GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps
- PMID: 12134080
- PMCID: PMC117324
- DOI: 10.1091/mbc.e02-02-0071
GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps
Abstract
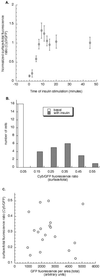
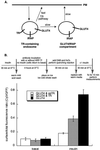
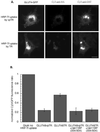
Insulin regulates glucose uptake into fat and muscle by modulating the distribution of the GLUT4 glucose transporter between the surface and interior of cells. The GLUT4 trafficking pathway overlaps with the general endocytic recycling pathway, but the degree and functional significance of the overlap are not known. In this study of intact adipocytes, we demonstrate, by using a compartment-specific fluorescence-quenching assay, that GLUT4 is equally distributed between two intracellular pools: the transferrin receptor-containing endosomes and a specialized compartment that excludes the transferrin receptor. These pools of GLUT4 are in dynamic communication with one another and with the cell surface. Insulin-induced redistribution of GLUT4 to the surface requires mobilization of both pools. These data establish a role for the general endosomal system in the specialized, insulin-regulated trafficking of GLUT4. Trafficking through the general endosomal system is regulated by rab11. Herein, we show that rab11 is required for the transport of GLUT4 from endosomes to the specialized compartment and for the insulin-induced translocation to the cell surface, emphasizing the importance of the general endosomal pathway in the specialized trafficking of GLUT4. Based on these findings we propose a two-step model for GLUT4 trafficking in which the general endosomal recycling compartment plays a specialized role in the insulin-regulated traffic of GLUT4. This compartment-based model provides the framework for understanding insulin-regulated trafficking at a molecular level.
Figures







Similar articles
-
Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles.Mol Biol Cell. 2001 Nov;12(11):3489-501. doi: 10.1091/mbc.12.11.3489. Mol Biol Cell. 2001. PMID: 11694583 Free PMC article.
-
Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3-L1 adipocytes.Mol Biol Cell. 1999 Nov;10(11):3675-88. doi: 10.1091/mbc.10.11.3675. Mol Biol Cell. 1999. PMID: 10564264 Free PMC article.
-
Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3-L1 adipocytes.Biochem J. 1996 Apr 15;315 ( Pt 2)(Pt 2):487-95. doi: 10.1042/bj3150487. Biochem J. 1996. PMID: 8615819 Free PMC article.
-
Compartment-ablation studies of GLUT4 distribution in adipocytes: evidence for multiple intracellular pools.Biochem Soc Trans. 1997 Aug;25(3):974-7. doi: 10.1042/bst0250974. Biochem Soc Trans. 1997. PMID: 9388584 Review.
-
Intracellular organization of insulin signaling and GLUT4 translocation.Recent Prog Horm Res. 2001;56:175-93. doi: 10.1210/rp.56.1.175. Recent Prog Horm Res. 2001. PMID: 11237212 Review.
Cited by
-
Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells.J Cell Biol. 2005 May 9;169(3):481-9. doi: 10.1083/jcb.200412069. Epub 2005 May 2. J Cell Biol. 2005. PMID: 15866888 Free PMC article.
-
Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160.Mol Biol Cell. 2004 Oct;15(10):4406-15. doi: 10.1091/mbc.e04-04-0333. Epub 2004 Jul 14. Mol Biol Cell. 2004. PMID: 15254270 Free PMC article.
-
Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor.Proc Natl Acad Sci U S A. 2011 Jun 21;108(25):10162-7. doi: 10.1073/pnas.1019268108. Epub 2011 Jun 6. Proc Natl Acad Sci U S A. 2011. PMID: 21646544 Free PMC article.
-
Development of a quantitative Correlative Light Electron Microscopy technique to study GLUT4 trafficking.Protoplasma. 2014 Mar;251(2):403-16. doi: 10.1007/s00709-013-0597-5. Epub 2014 Jan 4. Protoplasma. 2014. PMID: 24390248 Free PMC article.
-
Adenovirus Protein E4-ORF1 Activation of PI3 Kinase Reveals Differential Regulation of Downstream Effector Pathways in Adipocytes.Cell Rep. 2016 Dec 20;17(12):3305-3318. doi: 10.1016/j.celrep.2016.11.082. Cell Rep. 2016. PMID: 28009298 Free PMC article.
References
-
- Becker C, Sevilla L, Tomas E, Palacin M, Zorzano A, Fischer Y. The endosomal compartment is an insulin-sensitive recruitment site for GLUT4 and GLUT1 glucose transporters in cardiac myocytes. Endocrinology. 2001;142:5267–5276. - PubMed
-
- Cain CC, Trimble WS, Lienhard GE. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992;267:11681–11684. - PubMed
-
- Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

