Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells
- PMID: 12134082
- PMCID: PMC117326
- DOI: 10.1091/mbc.01-12-0589
Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells
Abstract
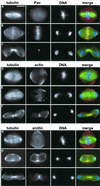
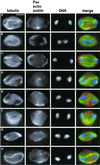
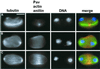
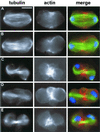
We have used double-stranded RNA-mediated interference (RNAi) to study Drosophila cytokinesis. We show that double-stranded RNAs for anillin, acGAP, pavarotti, rho1, pebble, spaghetti squash, syntaxin1A, and twinstar all disrupt cytokinesis in S2 tissue culture cells, causing gene-specific phenotypes. Our phenotypic analyses identify genes required for different aspects of cytokinesis, such as central spindle formation, actin accumulation at the cell equator, contractile ring assembly or disassembly, and membrane behavior. Moreover, the cytological phenotypes elicited by RNAi reveal simultaneous disruption of multiple aspects of cytokinesis. These phenotypes suggest interactions between central spindle microtubules, the actin-based contractile ring, and the plasma membrane, and lead us to propose that the central spindle and the contractile ring are interdependent structures. Finally, our results indicate that RNAi in S2 cells is a highly efficient method to detect cytokinetic genes, and predict that genome-wide studies using this method will permit identification of the majority of genes involved in Drosophila mitotic cytokinesis.
Figures



References
-
- Brill JA, Hime GR, Scharer-Schuksz M, Fuller MT. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development. 2000;127:3855–3864. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

