Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts
- PMID: 12134086
- PMCID: PMC117330
- DOI: 10.1091/mbc.01-11-0529
Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts
Abstract
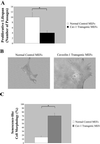
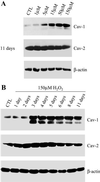
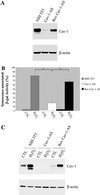
Caveolae are vesicular invaginations of the plasma membrane. Caveolin-1 is the principal structural component of caveolae in vivo. Several lines of evidence are consistent with the idea that caveolin-1 functions as a "transformation suppressor" protein. In fact, caveolin-1 mRNA and protein expression are lost or reduced during cell transformation by activated oncogenes. Interestingly, the human caveolin-1 gene is localized to a suspected tumor suppressor locus (7q31.1). We have previously demonstrated that overexpression of caveolin-1 arrests mouse embryonic fibroblasts in the G(0)/G(1) phase of the cell cycle through activation of a p53/p21-dependent pathway, indicating a role of caveolin-1 in mediating growth arrest. However, it remains unknown whether overexpression of caveolin-1 promotes cellular senescence in vivo. Here, we demonstrate that mouse embryonic fibroblasts transgenically overexpressing caveolin-1 show: 1) a reduced proliferative lifespan; 2) senescence-like cell morphology; and 3) a senescence-associated increase in beta-galactosidase activity. These results indicate for the first time that the expression of caveolin-1 in vivo is sufficient to promote and maintain the senescent phenotype. Subcytotoxic oxidative stress is known to induce premature senescence in diploid fibroblasts. Interestingly, we show that subcytotoxic level of hydrogen peroxide induces premature senescence in NIH 3T3 cells and increases endogenous caveolin-1 expression. Importantly, quercetin and vitamin E, two antioxidant agents, successfully prevent the premature senescent phenotype and the up-regulation of caveolin-1 induced by hydrogen peroxide. Also, we demonstrate that hydrogen peroxide alone, but not in combination with quercetin, stimulates the caveolin-1 promoter activity. Interestingly, premature senescence induced by hydrogen peroxide is greatly reduced in NIH 3T3 cells harboring antisense caveolin-1. Importantly, induction of premature senescence is recovered when caveolin-1 levels are restored. Taken together, these results clearly indicate a central role for caveolin-1 in promoting cellular senescence and they suggest the hypothesis that premature senescence may represent a tumor suppressor function mediated by caveolin-1 in vivo.
Figures









Similar articles
-
Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements.Cancer Res. 2006 Nov 15;66(22):10805-14. doi: 10.1158/0008-5472.CAN-06-1236. Cancer Res. 2006. PMID: 17108117 Free PMC article.
-
Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis.Arthritis Rheum. 2006 Mar;54(3):818-31. doi: 10.1002/art.21639. Arthritis Rheum. 2006. PMID: 16508959
-
Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism.Mol Biol Cell. 2001 Aug;12(8):2229-44. doi: 10.1091/mbc.12.8.2229. Mol Biol Cell. 2001. PMID: 11514613 Free PMC article.
-
Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints.Ann N Y Acad Sci. 2000 Jun;908:111-25. doi: 10.1111/j.1749-6632.2000.tb06640.x. Ann N Y Acad Sci. 2000. PMID: 10911952 Review.
-
Caveolin-1 in oncogenic transformation, cancer, and metastasis.Am J Physiol Cell Physiol. 2005 Mar;288(3):C494-506. doi: 10.1152/ajpcell.00458.2004. Am J Physiol Cell Physiol. 2005. PMID: 15692148 Review.
Cited by
-
Effect of quercetin and its metabolite on caveolin-1 expression induced by oxidized LDL and lysophosphatidylcholine in endothelial cells.J Clin Biochem Nutr. 2016 May;58(3):193-201. doi: 10.3164/jcbn.16-2. Epub 2016 Apr 16. J Clin Biochem Nutr. 2016. PMID: 27257344 Free PMC article.
-
Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration.Arthritis Res Ther. 2008;10(4):R87. doi: 10.1186/ar2468. Epub 2008 Aug 5. Arthritis Res Ther. 2008. PMID: 18681962 Free PMC article.
-
Caveolins in rhabdomyosarcoma.J Cell Mol Med. 2011 Dec;15(12):2553-68. doi: 10.1111/j.1582-4934.2011.01364.x. J Cell Mol Med. 2011. PMID: 21707915 Free PMC article. Review.
-
Caveolin-1 is a negative regulator of NADPH oxidase-derived reactive oxygen species.Free Radic Biol Med. 2014 Aug;73:201-13. doi: 10.1016/j.freeradbiomed.2014.04.029. Epub 2014 May 14. Free Radic Biol Med. 2014. PMID: 24835767 Free PMC article.
-
Loss of parkin promotes lipid rafts-dependent endocytosis through accumulating caveolin-1: implications for Parkinson's disease.Mol Neurodegener. 2015 Dec 1;10:63. doi: 10.1186/s13024-015-0060-5. Mol Neurodegener. 2015. PMID: 26627850 Free PMC article.
References
-
- Amundson SA, Myers TG, Fornace AJ., Jr Roles for p53 in growth arrest and apoptosis: putting on the brakes after genotoxic stress. Oncogene. 1998;17:3287–3299. - PubMed
-
- Black EJ, Clark W, Gillespie DA. Transient deactivation of ERK signaling is sufficient for stable entry into G0 in primary avian fibroblasts. Curr Biol. 2000;10:1119–1122. - PubMed
-
- Bumann J, Santo-Holtje L, Loffler H, Bamberg M, Rodemann HP. Radiation-induced alterations of the proliferation dynamics of human skin fibroblasts after repeated irradiation in the subtherapeutic dose range. Strahlenther Onkol. 1995;171:35–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

