Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta
- PMID: 12134160
- PMCID: PMC2387275
- DOI: 10.1038/ncb826
Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta
Abstract
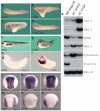
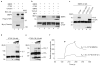
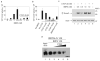
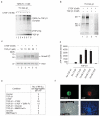
Connective-tissue growth factor (CTGF) is a secreted protein implicated in multiple cellular events including angiogenesis, skeletogenesis and wound healing. It is a member of the CCN family of secreted proteins, named after CTGF, cysteine-rich 61 (CYR61), and nephroblastoma overexpressed (NOV) proteins. The molecular mechanism by which CTGF or other CCN proteins regulate cell signalling is not known. CTGF contains a cysteine-rich domain (CR) similar to those found in chordin and other secreted proteins, which in some cases have been reported to function as bone morphogenetic protein (BMP) and TGF-beta binding domains. Here we show that CTGF directly binds BMP4 and TGF-beta 1 through its CR domain. CTGF can antagonize BMP4 activity by preventing its binding to BMP receptors and has the opposite effect, enhancement of receptor binding, on TGF-beta 1. These results show that CTGF inhibits BMP and activates TGF-beta signals by direct binding in the extracellular space.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

