Role of KIFC3 motor protein in Golgi positioning and integration
- PMID: 12135985
- PMCID: PMC2173137
- DOI: 10.1083/jcb.200202058
Role of KIFC3 motor protein in Golgi positioning and integration
Abstract
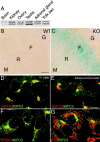
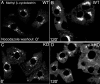
KIFC3, a microtubule (MT) minus end-directed kinesin superfamily protein, is expressed abundantly and is associated with the Golgi apparatus in adrenocortical cells. We report here that disruption of the kifC3 gene induced fragmentation of the Golgi apparatus when cholesterol was depleted. Analysis of the reassembly process of the Golgi apparatus revealed bidirectional movement of the Golgi fragments in both wild-type and kifC3-/- cells. However, we observed a markedly reduced inwardly directed motility of the Golgi fragments in cholesterol-depleted kifC3-/- cells compared with either cholesterol-depleted wild-type cells or cholesterol-replenished kifC3-/- cells. These results suggest that (a) under the cholesterol-depleted condition, reduced inwardly directed motility of the Golgi apparatus results in the observed Golgi scattering phenotype in kifC3-/- cells, and (b) cholesterol is necessary for the Golgi fragments to attain sufficient inwardly directed motility by MT minus end-directed motors other than KIFC3, such as dynein, in kifC3-/- cells. Furthermore, we showed that Golgi scattering was much more drastic in kifC3-/- cells than in wild-type cells to the exogenous dynamitin expression even in the presence of cholesterol. These results collectively demonstrate that KIFC3 plays a complementary role in Golgi positioning and integration with cytoplasmic dynein.
Figures








Similar articles
-
Identification of the kinesin KifC3 as a new player for positioning of peroxisomes and other organelles in mammalian cells.Biochim Biophys Acta. 2013 Dec;1833(12):3013-3024. doi: 10.1016/j.bbamcr.2013.08.002. Epub 2013 Aug 13. Biochim Biophys Acta. 2013. PMID: 23954441
-
KIFC3, a microtubule minus end-directed motor for the apical transport of annexin XIIIb-associated Triton-insoluble membranes.J Cell Biol. 2001 Oct 1;155(1):77-88. doi: 10.1083/jcb.200108042. J Cell Biol. 2001. PMID: 11581287 Free PMC article.
-
Motoring around the Golgi.Nat Cell Biol. 2002 Oct;4(10):E236-42. doi: 10.1038/ncb1002-e236. Nat Cell Biol. 2002. PMID: 12360306 Review.
-
Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells.J Cell Biol. 1994 Aug;126(3):661-75. doi: 10.1083/jcb.126.3.661. J Cell Biol. 1994. PMID: 8045931 Free PMC article.
-
Interaction of early secretory pathway and Golgi membranes with microtubules and microtubule motors.Biochemistry (Mosc). 2014 Sep;79(9):879-93. doi: 10.1134/S0006297914090053. Biochemistry (Mosc). 2014. PMID: 25385016 Review.
Cited by
-
KIFC3 promotes mitotic progression and integrity of the central spindle in cytokinesis.Cell Cycle. 2014;13(3):426-33. doi: 10.4161/cc.27266. Epub 2013 Nov 25. Cell Cycle. 2014. PMID: 24275865 Free PMC article.
-
Golgi positioning.Cold Spring Harb Perspect Biol. 2011 May 1;3(5):a005322. doi: 10.1101/cshperspect.a005322. Cold Spring Harb Perspect Biol. 2011. PMID: 21504874 Free PMC article. Review.
-
Knockdown of KIF15 suppresses proliferation of prostate cancer cells and induces apoptosis through PI3K/Akt signaling pathway.Cell Death Discov. 2023 Sep 1;9(1):326. doi: 10.1038/s41420-023-01625-5. Cell Death Discov. 2023. PMID: 37658042 Free PMC article.
-
A common pathomechanism in GMAP-210- and LBR-related diseases.JCI Insight. 2018 Dec 6;3(23):e121150. doi: 10.1172/jci.insight.121150. JCI Insight. 2018. PMID: 30518689 Free PMC article.
-
Transcriptional modulation of genes encoding structural characteristics of differentiating enterocytes during development of a polarized epithelium in vitro.Mol Biol Cell. 2007 Nov;18(11):4261-78. doi: 10.1091/mbc.e07-04-0308. Epub 2007 Aug 15. Mol Biol Cell. 2007. PMID: 17699590 Free PMC article.
References
-
- Allan, V.J., and T.A. Schroer. 1999. Membrane motors. Curr. Opin. Cell Biol. 11:476–482. - PubMed
-
- De Camilli, P., S.D. Emr, P.S. McPherson, and P. Novick. 1996. Phosphoinositides as regulators in membrane traffic. Science. 271:1533–1539. - PubMed
-
- Echard, A., F. Jollivet, O. Martinez, J.J. Lacapere, A. Rousselet, I. Janoueix-Lerosey, and B. Goud. 1998. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 279:580–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

