Brain monoglyceride lipase participating in endocannabinoid inactivation
- PMID: 12136125
- PMCID: PMC125056
- DOI: 10.1073/pnas.152334899
Brain monoglyceride lipase participating in endocannabinoid inactivation
Erratum in
- Proc Natl Acad Sci U S A 2002 Oct 15;99(21):13961
Abstract
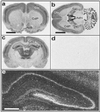
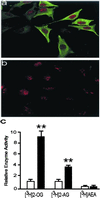
The endogenous cannabinoids (endocannabinoids) are lipid molecules that may mediate retrograde signaling at central synapses and other forms of short-range neuronal communication. The monoglyceride 2-arachidonoylglycerol (2-AG) meets several criteria of an endocannabinoid substance: (i) it activates cannabinoid receptors; (ii) it is produced by neurons in an activity-dependent manner; and (iii) it is rapidly eliminated. 2-AG inactivation is only partially understood, but it may occur by transport into cells and enzymatic hydrolysis. Here we tested the hypothesis that monoglyceride lipase (MGL), a serine hydrolase that converts monoglycerides to fatty acid and glycerol, participates in 2-AG inactivation. We cloned MGL by homology from a rat brain cDNA library. Its cDNA sequence encoded for a 303-aa protein with a calculated molecular weight of 33,367 daltons. Northern blot and in situ hybridization analyses revealed that MGL mRNA is heterogeneously expressed in the rat brain, with highest levels in regions where CB(1) cannabinoid receptors are also present (hippocampus, cortex, anterior thalamus, and cerebellum). Immunohistochemical studies in the hippocampus showed that MGL distribution has striking laminar specificity, suggesting a presynaptic localization of the enzyme. Adenovirus-mediated transfer of MGL cDNA into rat cortical neurons increased MGL expression and attenuated N-methyl-D-aspartate/carbachol-induced 2-AG accumulation in these cells. No such effect was observed on the accumulation of anandamide, another endocannabinoid lipid. The results suggest that hydrolysis by means of MGL is a primary mechanism for 2-AG inactivation in intact neurons.
Figures

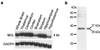


References
-
- Devane W. A., Hanus, L., Breuer, A., Pertwee, R. G., Stevenson, L. A., Griffin, G., Gibson, D., Mandelbaum, A., Etinger, A. & Mechoulam, R. (1992) Science 258, 1946-1949. - PubMed
-
- Sugiura T., Kondo, S., Sukagawa, A., Nakane, S., Shinoda, A., Itoh, K., Yamashita, A. & Waku, K. (1995) Biochem. Biophys. Res. Commun. 215, 89-97. - PubMed
-
- Mechoulam R., Ben-Shabat, S., Hanus, L., Ligumsky, M., Kaminski, N. E., Schatz, A. R., Gopher, A., Almog, S., Martin, B. R., Compton, D. R., et al. (1995) Biochem. Pharmacol. 50, 83-90. - PubMed
-
- Di Marzo V., Fontana, A., Cadas, H., Schinelli, S., Cimino, G., Schwartz, J. C. & Piomelli, D. (1994) Nature (London) 372, 686-691. - PubMed
-
- Stella N., Schweitzer, P. & Piomelli, D. (1997) Nature (London) 388, 773-778. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

