Multiple regulatory domains of IRF-5 control activation, cellular localization, and induction of chemokines that mediate recruitment of T lymphocytes
- PMID: 12138184
- PMCID: PMC133975
- DOI: 10.1128/MCB.22.16.5721-5740.2002
Multiple regulatory domains of IRF-5 control activation, cellular localization, and induction of chemokines that mediate recruitment of T lymphocytes
Abstract
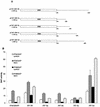
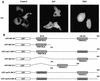
Transcription factors of the interferon regulatory factor (IRF) family have been identified as critical mediators of early inflammatory gene transcription in infected cells. We recently determined that, besides IRF-3 and IRF-7, IRF-5 serves as a direct transducer of virus-mediated signaling. In contrast to that mediated by the other two IRFs, IRF-5-mediated activation is virus specific. We show that, in addition to Newcastle disease virus (NDV) infection, vesicular stomatitis virus (VSV) and herpes simplex virus type 1 (HSV-1) infection activates IRF-5, leading to the induction of IFNA gene subtypes that are distinct from subtypes induced by NDV. The IRF-5-mediated stimulation of inflammatory genes is not limited to IFNA since in BJAB/IRF-5-expressing cells IRF-5 stimulates transcription of RANTES, macrophage inflammatory protein 1 beta, monocyte chemotactic protein 1, interleukin-8, and I-309 genes in a virus-specific manner. By transient- transfection assay, we identified constitutive-activation (amino acids [aa] 410 to 489) and autoinhibitory (aa 490 to 539) domains in the IRF-5 polypeptide. We identified functional nuclear localization signals (NLS) in the amino and carboxyl termini of IRF-5 and showed that both of these NLS are sufficient for nuclear translocation and retention in infected cells. Furthermore, we demonstrated that serine residues 477 and 480 play critical roles in the response to NDV infection. Mutation of these residues from serine to alanine dramatically decreased phosphorylation and resulted in a substantial loss of IRF-5 transactivation in infected cells. Thus, this study defines the regulatory phosphorylation sites that control the activity of IRF-5 in NDV-infected cells and provides further insight into the structure and function of IRF-5. It also shows that the range of IRF-5 immunoregulatory target genes includes members of the cytokine and chemokine superfamilies.
Figures
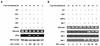
















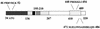
References
-
- Au, W. C., P. A. Moore, D. W. LaFleur, B. Tombal, and P. M. Pitha. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210-29217. - PubMed
-
- Au, W.-C., P. A. Moore, W. Lowther, Y.-T. Juang, and P. M. Pitha. 1995. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc. Natl. Acad. Sci. USA 92:11657-11661. - PMC - PubMed
-
- Au, W. C., and P. M. Pitha. 2001. Recruitment of multiple interferon regulatory factors and histone acetyltransferase to the transcriptionally active interferon a promoters. J. Biol. Chem. 276:41629-41637. - PubMed
-
- Au, W. C., W. S. Yeow, and P. M. Pitha. 2001. Analysis of functional domains of interferon regulatory factor 7 and its association with IRF-3. Virology 280:273-282. - PubMed
-
- Baggiolini, M., B. Dewald, and B. Moser. 1997. Human chemokines: an update. Annu. Rev. Immunol. 15:675-705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
