Functional evidence for retinoid X receptor (RXR) as a nonsilent partner in the thyroid hormone receptor/RXR heterodimer
- PMID: 12138189
- PMCID: PMC133993
- DOI: 10.1128/MCB.22.16.5782-5792.2002
Functional evidence for retinoid X receptor (RXR) as a nonsilent partner in the thyroid hormone receptor/RXR heterodimer
Abstract
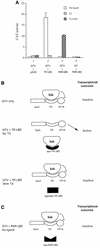
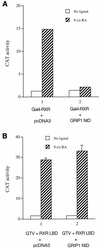
Many members of the thyroid hormone/retinoid receptor subfamily (type II nuclear receptors) function as heterodimers with the retinoid X receptor (RXR). In heterodimers which are referred to as permissive, such as peroxisome proliferator activated receptor/RXR, both partners can bind cognate ligands and elicit ligand-dependent transactivation. In contrast, the thyroid hormone receptor (TR)/RXR heterodimer is believed to be nonpermissive, where RXR is thought to be incapable of ligand binding and is often referred to as a silent partner. In this report, we used a sensitive derepression assay system that we developed previously to reexamine the TR/RXR interrelationship. We provide functional evidence suggesting that in a TR/RXR heterodimer, the RXR component can bind its ligand in vivo. Ligand binding by RXR does not appear to directly activate the TR/RXR heterodimer; instead, it leads to a (at least transient or dynamic) dissociation of a cellular inhibitor(s)/corepressor(s) from its TR partner and thus may serve to modulate unliganded TR-mediated repression and/or liganded TR-mediated activation. Our results argue against the current silent-partner model for RXR in the TR/RXR heterodimer and reveal an unexpected aspect of cross regulation between TR and RXR.
Figures
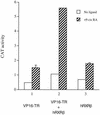
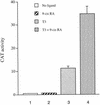

Similar articles
-
Novel roles of retinoid X receptor (RXR) and RXR ligand in dynamically modulating the activity of the thyroid hormone receptor/RXR heterodimer.J Biol Chem. 2004 Feb 27;279(9):7427-37. doi: 10.1074/jbc.M311596200. Epub 2003 Dec 10. J Biol Chem. 2004. PMID: 14668324
-
A permissive retinoid X receptor/thyroid hormone receptor heterodimer allows stimulation of prolactin gene transcription by thyroid hormone and 9-cis-retinoic acid.Mol Cell Biol. 2004 Jan;24(2):502-13. doi: 10.1128/MCB.24.2.502-513.2004. Mol Cell Biol. 2004. PMID: 14701725 Free PMC article.
-
The ligand-binding domains of the thyroid hormone/retinoid receptor gene subfamily function in vivo to mediate heterodimerization, gene silencing, and transactivation.Mol Cell Biol. 1995 Mar;15(3):1817-25. doi: 10.1128/MCB.15.3.1817. Mol Cell Biol. 1995. PMID: 7862171 Free PMC article.
-
Natural and synthetic retinoid X receptor ligands and their role in selected nuclear receptor action.Biochimie. 2020 Dec;179:157-168. doi: 10.1016/j.biochi.2020.09.027. Epub 2020 Oct 1. Biochimie. 2020. PMID: 33011201 Review.
-
Thyroid hormone and thyroid hormone nuclear receptors: History and present state of art.Endocr Regul. 2021 May 21;55(2):103-119. doi: 10.2478/enr-2021-0012. Endocr Regul. 2021. PMID: 34020531 Review.
Cited by
-
Modeling, synthesis, and biological evaluation of potential retinoid X receptor (RXR) selective agonists: novel analogues of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) and (E)-3-(3-(1,2,3,4-tetrahydro-1,1,4,4,6-pentamethylnaphthalen-7-yl)-4-hydroxyphenyl)acrylic acid (CD3254).J Med Chem. 2013 Nov 14;56(21):8432-54. doi: 10.1021/jm4008517. Epub 2013 Nov 1. J Med Chem. 2013. PMID: 24180745 Free PMC article.
-
Integrating Thyroid Hormone Signaling in Hypothalamic Control of Metabolism: Crosstalk Between Nuclear Receptors.Int J Mol Sci. 2018 Jul 11;19(7):2017. doi: 10.3390/ijms19072017. Int J Mol Sci. 2018. PMID: 29997323 Free PMC article. Review.
-
Identification of thyroid hormone receptor active compounds using a quantitative high-throughput screening platform.Curr Chem Genom Transl Med. 2014 Mar 7;8:36-46. doi: 10.2174/2213988501408010036. eCollection 2014. Curr Chem Genom Transl Med. 2014. PMID: 24772387 Free PMC article.
-
Inhibition of testicular embryonal carcinoma cell tumorigenicity by peroxisome proliferator-activated receptor-β/δ- and retinoic acid receptor-dependent mechanisms.Oncotarget. 2015 Nov 3;6(34):36319-37. doi: 10.18632/oncotarget.5415. Oncotarget. 2015. PMID: 26431381 Free PMC article.
-
Transcriptional modulations by RXR agonists are only partially subordinated to PPARalpha signaling and attest additional, organ-specific, molecular cross-talks.Gene Expr. 2005;12(3):177-92. doi: 10.3727/000000005783992098. Gene Expr. 2005. PMID: 16128002 Free PMC article.
References
-
- Aranda, A., and A. Pascual. 2001. Nuclear hormone receptors and gene expression. Physiol. Rev. 81:1269-1304. - PubMed
-
- Baniahmad, A., C. Steiner, A. C. Kohne, and R. Renkawitz. 1990. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell 61:505-514. - PubMed
-
- Blumberg, B., and R. M. Evans. 1998. Orphan nuclear receptors-new ligands and new possibilities. Genes Dev. 12:3149-3155. - PubMed
-
- Brzozowski, A. M., A. C. Pike, Z. Dauter, R. E. Hubbard, T. Bonn, O. Engstrom, L. Ohman, G. L. Greene, J. A. Gustafsson, and M. Carlquist. 1997. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753-758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
