Interactions between telomerase and primase physically link the telomere and chromosome replication machinery
- PMID: 12138196
- PMCID: PMC133977
- DOI: 10.1128/MCB.22.16.5859-5868.2002
Interactions between telomerase and primase physically link the telomere and chromosome replication machinery
Abstract
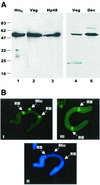
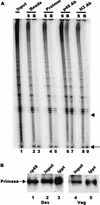
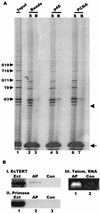
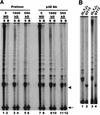
In the ciliate Euplotes crassus, millions of new telomeres are synthesized by telomerase and polymerase alpha-primase during macronuclear development in mated cells. Concomitant with de novo telomere formation, telomerase assembles into higher-order complexes of 550 kDa, 1,600 kDa, and 5 MDa. We show here that telomerase is physically associated with the lagging-strand replication machinery in these complexes. Antibodies against DNA primase precipitated telomerase activity from all three complexes from mated cells but not the 280-kDa telomerase complex from vegetatively growing cells. Moreover, when telomerase was affinity purified, primase copurified with enzyme from mated cells but not with the 280-kDa vegetative complex. Thus, the association of telomerase and primase is developmentally regulated. Intriguingly, PCNA (proliferating cell nuclear antigen) was also found in the 5-MDa complex from mated cells. We therefore speculate that this complex is a complete telomere synthesis machine, while the smaller complexes are assembly intermediates. The physical association of telomerase and primase explains the coordinate regulation of telomeric G- and C-strand synthesis and the efficiency of telomere addition in E. crassus.
Figures



Similar articles
-
Developmentally programmed assembly of higher order telomerase complexes with distinct biochemical and structural properties.Genes Dev. 1998 Sep 15;12(18):2921-31. doi: 10.1101/gad.12.18.2921. Genes Dev. 1998. PMID: 9744868 Free PMC article.
-
Developmentally regulated initiation of DNA synthesis by telomerase: evidence for factor-assisted de novo telomere formation.EMBO J. 1997 May 1;16(9):2507-18. doi: 10.1093/emboj/16.9.2507. EMBO J. 1997. PMID: 9171363 Free PMC article.
-
Telomere C-Strand Fill-In Machinery: New Insights into the Human CST-DNA Polymerase Alpha-Primase Structures and Functions.Subcell Biochem. 2024;104:73-100. doi: 10.1007/978-3-031-58843-3_5. Subcell Biochem. 2024. PMID: 38963484 Review.
-
CST-polymerase α-primase solves a second telomere end-replication problem.Nature. 2024 Mar;627(8004):664-670. doi: 10.1038/s41586-024-07137-1. Epub 2024 Feb 28. Nature. 2024. PMID: 38418884 Free PMC article.
-
CST-Polα/Primase: the second telomere maintenance machine.Genes Dev. 2023 Jul 1;37(13-14):555-569. doi: 10.1101/gad.350479.123. Epub 2023 Jul 26. Genes Dev. 2023. PMID: 37495394 Free PMC article. Review.
Cited by
-
Dysfunction of chromatin assembly factor 1 induces shortening of telomeres and loss of 45S rDNA in Arabidopsis thaliana.Plant Cell. 2010 Aug;22(8):2768-80. doi: 10.1105/tpc.110.076182. Epub 2010 Aug 10. Plant Cell. 2010. PMID: 20699390 Free PMC article.
-
Alterations of DNA and chromatin structures at telomeres and genetic instability in mouse cells defective in DNA polymerase alpha.Mol Cell Biol. 2005 Dec;25(24):11073-88. doi: 10.1128/MCB.25.24.11073-11088.2005. Mol Cell Biol. 2005. PMID: 16314528 Free PMC article.
-
Pol12, the B subunit of DNA polymerase alpha, functions in both telomere capping and length regulation.Genes Dev. 2004 May 1;18(9):992-1006. doi: 10.1101/gad.300004. Genes Dev. 2004. PMID: 15132993 Free PMC article.
-
Ku is required for telomeric C-rich strand maintenance but not for end-to-end chromosome fusions in Arabidopsis.Proc Natl Acad Sci U S A. 2003 Jan 21;100(2):611-5. doi: 10.1073/pnas.0236128100. Epub 2003 Jan 2. Proc Natl Acad Sci U S A. 2003. PMID: 12511598 Free PMC article.
-
Telomere replication: poised but puzzling.J Cell Mol Med. 2011 Jan;15(1):3-13. doi: 10.1111/j.1582-4934.2010.01220.x. J Cell Mol Med. 2011. PMID: 21122064 Free PMC article. Review.
References
-
- Arezi, B., and R. D. Kuchta. 2000. Eukaryotic DNA primase. Trends Biochem. Sci. 25:572-576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
