Molecular anatomy of the human excision nuclease assembled at sites of DNA damage
- PMID: 12138203
- PMCID: PMC133982
- DOI: 10.1128/MCB.22.16.5938-5945.2002
Molecular anatomy of the human excision nuclease assembled at sites of DNA damage
Abstract
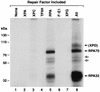
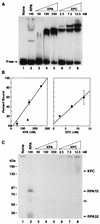
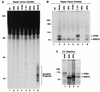
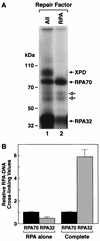
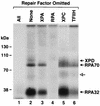
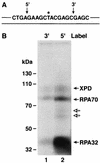
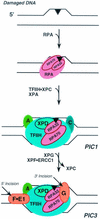
Human nucleotide excision repair is initiated by six repair factors (XPA, RPA, XPC-HR23B, TFIIH, XPF-ERCC1, and XPG) which sequentially assemble at sites of DNA damage and effect excision of damage-containing oligonucleotides. We here describe the molecular anatomy of the human excision nuclease assembled at the site of a psoralen-adducted thymine. Three polypeptides, primarily positioned 5' to the damage, are in close physical proximity to the psoralen lesion and thus are cross-linked to the damaged DNA: these proteins are RPA70, RPA32, and the XPD subunit of TFIIH. While both XPA and XPC bind damaged DNA and are required for XPD cross-linking to the psoralen-adducted base, neither XPA nor XPC is cross-linked to the psoralen adduct. The presence of other repair factors, in particular TFIIH, alters the mode of RPA binding and the position of its subunits relative to the psoralen lesion. Based on these results, we propose that RPA70 makes the initial contact with psoralen-damaged DNA but that within preincision complexes, it is RPA32 and XPD that are in close contact with the lesion.
Figures

References
-
- Chang, W.-H., and R. D. Kornberg. 2000. Electron crystal structure of the transcription factor and DNA repair complex, core TFIIH. Cell 102:609-613. - PubMed
-
- Cimino, G. D., H. B. Gamper, S. T. Isaacs, and J. E. Hearst. 1985. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu. Rev. Biochem. 54:1151-1193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
