Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure
- PMID: 12140368
- PMCID: PMC124946
- DOI: 10.1073/pnas.162356199
Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure
Abstract
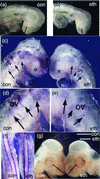
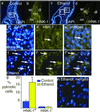
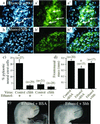
Alcohol is a teratogen that induces a variety of abnormalities including brain and facial defects [Jones, K. & Smith, D. (1973) Lancet 2, 999-1001], with the exact nature of the deficit depending on the time and magnitude of the dose of ethanol to which developing fetuses are exposed. In addition to abnormal facial structures, ethanol-treated embryos exhibit a highly characteristic pattern of cell death. Dying cells are observed in the premigratory and migratory neural crest cells that normally populate most facial structures. The observation that blocking Sonic hedgehog (Shh) signaling results in similar craniofacial abnormalities prompted us to examine whether there was a link between this aspect of fetal alcohol syndrome and loss of Shh. We demonstrate that administration of ethanol to chick embryos results in a dramatic loss of Shh, as well as a loss of transcripts involved in Shh signaling pathways. In contrast, other signaling molecules examined do not demonstrate such dramatic changes. Furthermore, we demonstrate that both the ethanol-induced cranial neural crest cell death and the associated craniofacial growth defect can be rescued by application of Shh. These data suggest that craniofacial anomalies resulting from fetal alcohol exposure are caused at least partially by loss of Shh and subsequent neural crest cell death.
Figures


References
-
- Ahlgren S. C. & Bronner-Fraser, M. (1999) Curr. Biol. 9, 1304-1314. - PubMed
-
- Hu D. & Helms, J. A. (1999) Development (Cambridge, U.K.) 126, 4873-4884. - PubMed
-
- Chiang C., Litingtung, Y., Lee, E., Young, K. E., Corden, J. L., Westphal, H. & Beachy, P. A. (1996) Nature (London) 383, 407-413. - PubMed
-
- Roessler E., Belloni, E., Gaudenz, K., Jay, P., Scherer, S. W., Tsui, L.-C. & Muenke, M. (1996) Nat. Genet. 14, 357-360. - PubMed
-
- Echelard Y., Epstein, D. J., St.-Jacques, B., Shen, L., Mohler, J., McMahon, J. A. & McMahon, A. P. (1993) Cell 75, 1417-1430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

