ZntB is a novel Zn2+ transporter in Salmonella enterica serovar Typhimurium
- PMID: 12142406
- PMCID: PMC135258
- DOI: 10.1128/JB.184.16.4369-4373.2002
ZntB is a novel Zn2+ transporter in Salmonella enterica serovar Typhimurium
Abstract
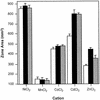
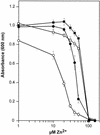
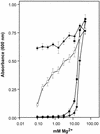
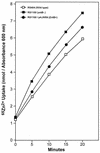
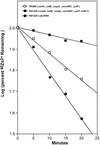
A Zn2+ transport system encoded by the zntB locus of Salmonella enterica serovar Typhimurium has been identified. The protein encoded by this locus is homologous to the CorA family of Mg2+ transport proteins and is widely distributed among the eubacteria. Mutations at zntB confer an increased sensitivity to the cytotoxic effects of Zn2+ and Cd2+, a phenotype that suggests that the encoded protein mediates the efflux of both cations. A direct analysis of transport activity identified a capacity for Zn2+ efflux. These data identify ZntB as a zinc efflux pathway in the enteric bacteria and assign a new function to the CorA family of cation transporters.
Figures
Similar articles
-
Membrane topology of the ZntB efflux system of Salmonella enterica serovar Typhimurium.J Bacteriol. 2003 Jan;185(1):374-6. doi: 10.1128/JB.185.1.374-376.2003. J Bacteriol. 2003. PMID: 12486076 Free PMC article.
-
X-ray crystallography and isothermal titration calorimetry studies of the Salmonella zinc transporter ZntB.Structure. 2011 May 11;19(5):700-10. doi: 10.1016/j.str.2011.02.011. Structure. 2011. PMID: 21565704 Free PMC article.
-
Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system.Mol Microbiol. 1991 Nov;5(11):2753-62. doi: 10.1111/j.1365-2958.1991.tb01984.x. Mol Microbiol. 1991. PMID: 1779764
-
Microbial magnesium transport: unusual transporters searching for identity.Mol Microbiol. 1998 Apr;28(2):217-26. doi: 10.1046/j.1365-2958.1998.00810.x. Mol Microbiol. 1998. PMID: 9622348 Review.
-
Magnesium transporters: properties, regulation and structure.Front Biosci. 2006 Sep 1;11:3149-63. doi: 10.2741/2039. Front Biosci. 2006. PMID: 16720382 Review.
Cited by
-
Cation permeability in CorA family of proteins.Sci Rep. 2020 Jan 21;10(1):840. doi: 10.1038/s41598-020-57869-z. Sci Rep. 2020. PMID: 31965019 Free PMC article.
-
Common functions or only phylogenetically related? The large family of PLAC8 motif-containing/PCR genes.Mol Cells. 2011 Jan;31(1):1-7. doi: 10.1007/s10059-011-0024-8. Epub 2011 Jan 6. Mol Cells. 2011. PMID: 21347707 Free PMC article. Review.
-
Structures, Mechanisms, and Physiological Functions of Zinc Transporters in Different Biological Kingdoms.Int J Mol Sci. 2024 Mar 6;25(5):3045. doi: 10.3390/ijms25053045. Int J Mol Sci. 2024. PMID: 38474291 Free PMC article. Review.
-
Extreme zinc tolerance in acidophilic microorganisms from the bacterial and archaeal domains.Extremophiles. 2013 Jan;17(1):75-85. doi: 10.1007/s00792-012-0495-3. Epub 2012 Nov 10. Extremophiles. 2013. PMID: 23143658
-
Mycobacteria, metals, and the macrophage.Immunol Rev. 2015 Mar;264(1):249-63. doi: 10.1111/imr.12265. Immunol Rev. 2015. PMID: 25703564 Free PMC article. Review.
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. A. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Wiley-Interscience, New York, N.Y.
-
- Beard, S. J., R. Hashim, J. Membrillo-Hernandez, M. N. Hughes, and R. K. Poole. 1997. Zinc(II) tolerance in Escherichia coli K-12: evidence that the zntA gene (o732) encodes a cation transport ATPase. Mol. Microbiol. 25:883-891. - PubMed
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Brocklehurst, K. R., J. L. Hobman, B. Lawley, L. Blank, S. J. Marshall, N. L. Brown, and A. P. Morby. 1999. ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol. Microbiol. 31:893-902. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

