Role of the C-terminal domain of the alpha subunit of RNA polymerase in LuxR-dependent transcriptional activation of the lux operon during quorum sensing
- PMID: 12142422
- PMCID: PMC135237
- DOI: 10.1128/JB.184.16.4520-4528.2002
Role of the C-terminal domain of the alpha subunit of RNA polymerase in LuxR-dependent transcriptional activation of the lux operon during quorum sensing
Abstract
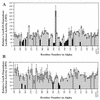
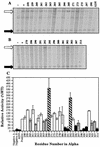
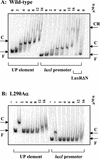
During quorum sensing in Vibrio fischeri, the luminescence, or lux, operon is regulated in a cell density-dependent manner by the activator LuxR in the presence of an acylated homoserine lactone autoinducer molecule [N-(3-oxohexanoyl) homoserine lactone]. LuxR, which binds to the lux operon promoter at a position centered at -42.5 relative to the transcription initiation site, is thought to function as an ambidextrous activator making multiple contacts with RNA polymerase (RNAP). The specific role of the alpha-subunit C-terminal domain (alphaCTD) of RNAP in LuxR-dependent transcriptional activation of the lux operon promoter has been investigated. The effects of 70 alanine substitution variants of the alpha subunit were determined in vivo by measuring the rate of transcription of the lux operon via luciferase assays in recombinant Escherichia coli. The mutant RNAPs from strains exhibiting at least twofold-increased or -decreased activity in comparison to the wild type were further examined by in vitro assays. Since full-length LuxR has not been purified, an autoinducer-independent N-terminally truncated form of LuxR, LuxRDeltaN, was used for in vitro studies. Single-round transcription assays were performed using reconstituted mutant RNAPs in the presence of LuxRDeltaN, and 14 alanine substitutions in the alphaCTD were identified as having negative effects on the rate of transcription from the lux operon promoter. Five of these 14 alpha variants were also involved in the mechanisms of both LuxR- and LuxRDeltaN-dependent activation in vivo. The positions of these residues lie roughly within the 265 and 287 determinants in alpha that have been identified through studies of the cyclic AMP receptor protein and its interactions with RNAP. This suggests a model where residues 262, 265, and 296 in alpha play roles in DNA recognition and residues 290 and 314 play roles in alpha-LuxR interactions at the lux operon promoter during quorum sensing.
Figures


Similar articles
-
Involvement of region 4 of the sigma70 subunit of RNA polymerase in transcriptional activation of the lux operon during quorum sensing.FEMS Microbiol Lett. 2003 Nov 21;228(2):193-201. doi: 10.1016/S0378-1097(03)00750-X. FEMS Microbiol Lett. 2003. PMID: 14638424
-
Involvement of the RNA polymerase alpha-subunit C-terminal domain in LuxR-dependent activation of the Vibrio fischeri luminescence genes.J Bacteriol. 1999 Aug;181(15):4704-7. doi: 10.1128/JB.181.15.4704-4707.1999. J Bacteriol. 1999. PMID: 10419977 Free PMC article.
-
Amino acid residues in LuxR critical for its mechanism of transcriptional activation during quorum sensing in Vibrio fischeri.J Bacteriol. 2001 Jan;183(1):387-92. doi: 10.1128/JB.183.1.387-392.2001. J Bacteriol. 2001. PMID: 11114940 Free PMC article.
-
Quorum regulation of luminescence in Vibrio fischeri.J Mol Microbiol Biotechnol. 1999 Aug;1(1):5-12. J Mol Microbiol Biotechnol. 1999. PMID: 10941779 Review.
-
Transcriptional activation of the catechol and chlorocatechol operons: variations on a theme.Gene. 1998 Nov 26;223(1-2):257-67. doi: 10.1016/s0378-1119(98)00366-7. Gene. 1998. PMID: 9858745 Review.
Cited by
-
Structure/function analysis of the Pantoea stewartii quorum-sensing regulator EsaR as an activator of transcription.J Bacteriol. 2009 Dec;191(24):7402-9. doi: 10.1128/JB.00994-09. Epub 2009 Oct 9. J Bacteriol. 2009. PMID: 19820098 Free PMC article.
-
Directed evolution of Vibrio fischeri LuxR for improved response to butanoyl-homoserine lactone.Appl Environ Microbiol. 2007 Sep;73(18):5775-81. doi: 10.1128/AEM.00060-07. Epub 2007 Aug 3. Appl Environ Microbiol. 2007. PMID: 17675429 Free PMC article.
-
Transcription control engineering and applications in synthetic biology.Synth Syst Biotechnol. 2017 Oct 4;2(3):176-191. doi: 10.1016/j.synbio.2017.09.003. eCollection 2017 Sep. Synth Syst Biotechnol. 2017. PMID: 29318198 Free PMC article. Review.
-
N- and C-terminal regions of the quorum-sensing activator TraR cooperate in interactions with the alpha and sigma-70 components of RNA polymerase.Mol Microbiol. 2009 Oct;74(2):330-46. doi: 10.1111/j.1365-2958.2009.06865.x. Epub 2009 Sep 2. Mol Microbiol. 2009. PMID: 19732344 Free PMC article.
-
Engineering of Bacillus Promoters Based on Interacting Motifs between UP Elements and RNA Polymerase (RNAP) α-Subunit.Int J Mol Sci. 2022 Nov 3;23(21):13480. doi: 10.3390/ijms232113480. Int J Mol Sci. 2022. PMID: 36362266 Free PMC article.
References
-
- Aiyar, S. E., S. M. McLeod, W. Ross, C. A. Harvanen, M. S. Thomas, R. C. Johnson, and R. L. Gourse. 2002. Architecture of Fis-activated transcription complexes at the Escherichia coli rrnB P1 and rrnE P1 promoters. J. Mol. Biol. 316:501-516. - PubMed
-
- Blatter, E. E., W. Ross, H. Tang, R. L. Gourse, and R. H. Ebright. 1994. Domain organization of RNA polymerase α subunit: C-terminal 85 amino acids constitute an independently folded domain capable of dimerization and DNA binding. Cell 78:889-896. - PubMed
-
- Bokal, A. J., W. Ross, and R. L. Gourse. 1995. The transcriptional activator protein FIS: DNA interactions and cooperative interactions with RNA polymerase at the Escherichia coli rrnB P1 promoter. J. Mol. Biol. 245:197-207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

