[2,4-13 C2 ]-beta-Hydroxybutyrate metabolism in human brain
- PMID: 12142574
- PMCID: PMC2995543
- DOI: 10.1097/00004647-200207000-00014
[2,4-13 C2 ]-beta-Hydroxybutyrate metabolism in human brain
Abstract
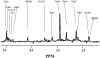
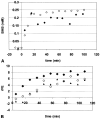
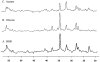
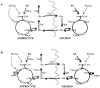
Infusions of [2,4-13C2]-beta-hydroxybutyrate and 1H-13C polarization transfer spectroscopy were used in normal human subjects to detect the entry and metabolism of beta-hydroxybutyrate in the brain. During the 2-hour infusion study, 13C label was detectable in the beta-hydroxybutyrate resonance positions and in the amino acid pools of glutamate, glutamine, and aspartate. With a plasma concentration of 2.25 +/- 0.24 mmol/L (four volunteers), the apparent tissue beta-hydroxybutyrate concentration reached 0.18 +/- 0.06 mmol/L during the last 20 minutes of the study. The relative fractional enrichment of 13C-4-glutamate labeling was 6.78 +/- 1.71%, whereas 13C-4-glutamine was 5.68 +/- 1.84%. Steady-state modeling of the 13C label distribution in glutamate and glutamine suggests that, under these conditions, the consumption of the beta-hydroxybutyrate is predominantly neuronal, used at a rate of 0.032 +/- 0.009 mmol. kg-1. min-1, and accounts for 6.4 +/- 1.6% of total acetyl coenzyme A oxidation. These results are consistent with minimal accumulation of cerebral ketones with rapid utilization, implying blood-brain barrier control of ketone oxidation in the nonfasted adult human brain.
Figures
References
-
- Aoki C, Kaneko T, Starr A, Pickel VM. Identification of mitochondrial and non-mitochondrial glutaminase within select neurons and glia of rat forebrain by electron microscopic immunocytochemistry. J Neurosci Res. 1991;28:531–548. - PubMed
-
- Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes and exercise. Diabetes Metab Rev. 1989;5:247–270. - PubMed
-
- Blomqvist G, Thorell JO, Ingvar M, Grill V, Widen L, Stone-Elander S. Use of R-β-[1-11C]-hydroxybutyrate in PET studies of regional cerebral uptake of ketone bodies in humans. Am J Physiol. 1995;269:E948–959. - PubMed
-
- Gjedde A, Crone C. Induction processes in blood-brain transfer of ketone bodies during starvation. Am J Physiol. 1975;229:1165–1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

