Characterization of G proteins involved in activation of nonselective cation channels by endothelin(B) receptor
- PMID: 12145101
- PMCID: PMC1573433
- DOI: 10.1038/sj.bjp.0704805
Characterization of G proteins involved in activation of nonselective cation channels by endothelin(B) receptor
Abstract
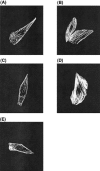
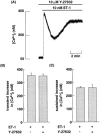
1: We recently demonstrated that endothelin-1 (ET-1) activates two types of Ca(2+)-permeable nonselective cation channels (NSCC-1 and NSCC-2) in Chinese hamster ovary cells expressing endothelin(B) receptors (CHO-ET(B)R) that couple with G(q) and G(i). The purpose of the present study was to identify the G proteins involved in the activation of these Ca(2+) channels by ET-1. For this purpose, we constructed CHO cells expressing an unpalmitoylated (Cys(402)Cys(403) Cys(405)-->Ser(402)Ser(403)Ser(405)) ET(B)R (CHO-SerET(B)R) and ET(B)R truncated at the cytoplasmic tail downstream of Cys(403) (CHO-ET(B)RDelta403). 2: Based on the data obtained from actin stress fibre formation, CHO-ET(B)R couple with G(13). Therefore, CHO-ET(B)R couple with G(q), G(i) and G(13). CHO-SerET(B)R and CHO-ET(B)RDelta403 couple with G(13) and G(q), respectively. 3: ET-1 activated NSCC-1 in CHO-ET(B)R preincubated with phospholipase C (PLC) inhibitor, U73122, and in CHO-SerET(B)R. On the other hand, ET-1 failed to activate Ca(2+) channels in CHO-ET(B)RDelta403. Microinjection of dominant negative mutants of G(13) (G(13)G225A) abolished activation of NSCC-1 and NSCC-2 in CHO-ET(B)R and that of NSCC-1 in CHO-SerET(B)R. 4: Y-27632, a specific Rho-associated kinase (ROCK) inhibitor, did not affect the ET-1-induced transient and sustained increase in [Ca(2+)](i) in CHO-ET(B)R. 5: These results indicate that (1) the cytoplasmic tail downstream of the palmitoylation sites of ET(B)R, but not the palmitoylation site itself, is essential for coupling with G(13), (2) the activation mechanism of each Ca(2+) channel by ET-1 is different in CHO-ET(B)R. NSCC-1 activation depends on G(13)-dependent cascade, and NSCC-2 activation depends on both G(q)/PLC- and G(13)-dependent cascades. Moreover, ROCK-dependent cascade is not involved in the activation of these channels.
Figures






Similar articles
-
Molecular mechanisms for the activation of voltage-independent Ca2+ channels by endothelin-1 in chinese hamster ovary cells stably expressing human endothelin(A) receptors.Mol Pharmacol. 2002 Jul;62(1):75-80. doi: 10.1124/mol.62.1.75. Mol Pharmacol. 2002. PMID: 12065757
-
Molecular mechanisms for activation of voltage-independent Ca2+ channels by endothelin-1/endothelin-A receptors.J Cardiovasc Pharmacol. 2004 Nov;44 Suppl 1:S219-23. doi: 10.1097/01.fjc.0000166252.66486.06. J Cardiovasc Pharmacol. 2004. PMID: 15838284
-
Molecular mechanism for endothelin-1-induced stress-fiber formation: analysis of G proteins using a mutant endothelin(A) receptor.Mol Pharmacol. 2002 Feb;61(2):277-84. doi: 10.1124/mol.61.2.277. Mol Pharmacol. 2002. PMID: 11809851
-
Involvement of extracellular Ca2+ influx through voltage-independent Ca2+ channels in endothelin-1 function.Cell Signal. 2005 Aug;17(8):911-6. doi: 10.1016/j.cellsig.2005.01.001. Epub 2005 Feb 16. Cell Signal. 2005. PMID: 15894164 Review.
-
Ca2+ entry channels in rat thoracic aortic smooth muscle cells activated by endothelin-1.Jpn J Pharmacol. 1999 Aug;80(4):281-8. doi: 10.1254/jjp.80.281. Jpn J Pharmacol. 1999. PMID: 10496327 Review.
Cited by
-
Differences in Endothelin B Receptor Isoforms Expression and Function in Breast Cancer Cells.J Cancer. 2020 Feb 19;11(9):2688-2701. doi: 10.7150/jca.41004. eCollection 2020. J Cancer. 2020. PMID: 32201539 Free PMC article.
-
Endothelin-1 activation of ETB receptors leads to a reduced cellular proliferative rate and an increased cellular footprint.Exp Cell Res. 2012 Jun 10;318(10):1125-33. doi: 10.1016/j.yexcr.2012.03.029. Epub 2012 Apr 4. Exp Cell Res. 2012. PMID: 22504006 Free PMC article.
-
Hyperforin activates nonselective cation channels (NSCCs).Br J Pharmacol. 2005 May;145(1):75-83. doi: 10.1038/sj.bjp.0706155. Br J Pharmacol. 2005. PMID: 15723093 Free PMC article.
References
-
- ARAI H., HORI S., ARAMORI I., OHKUBO H., NAKANISHI S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. - PubMed
-
- ARAMORI I., NAKANISHI S. Coupling of two endothelin receptor subtypes to differing signal transduction in transfected Chinese hamster ovary cells. J. Biol. Chem. 1992;267:12468–12474. - PubMed
-
- BETUING S., DAVIAUD D., PAGES C., BONNARD E., VALET P., LAFONTAN M., SAULNIER-BLACHE J.S. Gβγ-independent coupling of α2-adrenergic receptor to p21rhoA in preadipocytes. J. Biol. Chem. 1998;273:15804–15810. - PubMed
-
- BUHL A.M., JOHNSON N.L., DHANASEKARAN N., JOHNSON G.L. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 1995;270:24631–24634. - PubMed
-
- GOHLA A., OFFERMANNS S., WILKIE T.M., SCHULTZ G. Differential involvement of Galpha12 and Galpha13 in receptor-mediated stress fiber formation. J. Biol. Chem. 1999;274:17901–17907. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

