Ebselen attenuates oxidative stress-induced apoptosis via the inhibition of the c-Jun N-terminal kinase and activator protein-1 signalling pathway in PC12 cells
- PMID: 12145102
- PMCID: PMC1573436
- DOI: 10.1038/sj.bjp.0704808
Ebselen attenuates oxidative stress-induced apoptosis via the inhibition of the c-Jun N-terminal kinase and activator protein-1 signalling pathway in PC12 cells
Abstract
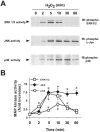
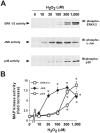
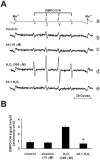
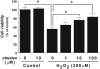
1: Ebselen (2-phenyl-1,2-benzisoselenazol-3[2H]-one) is a selenoorganic compound exhibiting both glutathione peroxidase activity and antioxidant activity. Although it has been reported that ebselen is effective for oxidative stress-induced neuronal damage both in vivo and clinically, the precise mechanisms of the efficacy have not yet been elucidated. Thus, we hypothesized that ebselen may affect reactive oxygen species-induced mitogen-activated protein (MAP) kinase activation in cultured PC12 cells. 2: Our findings showed that hydrogen peroxide (H(2)O(2)) stimulated rapid and significant activation of extracellular signal-regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK) and p38 in PC12 cells, which is a model of catecholamine-containing neurons. 3: H(2)O(2)-induced JNK activation was inhibited by ebselen, whereas ERK1/2 and p38 activation by H(2)O(2) were not affected by ebselen. 4: Inhibition by ebselen of H(2)O(2)-induced hydroxyl radical generation in PC12 cells was observed using electron paramagnetic resonance measurements. Ebselen also inhibited H(2)O(2)-induced increases in DNA binding activity of activator protein-1 (AP-1), a downstream transcription factor of JNK, composed of the c-Jun homo/heterodimer. 5: Finally, pretreatment of cells with ebselen resulted in a significant recovery from cell death including apoptosis by H(2)O(2) in PC12 cells. 6 These findings suggest that ebselen attenuates oxidative stress-induced neuronal cell death through the inhibition of the JNK and AP-1 signalling pathway. Thus, inhibition of JNK by ebselen may imply its usefulness for treatment of ischaemic cerebral diseases relevant to neuronal cell death.
Figures



Similar articles
-
Ebselen inhibits p38 mitogen-activated protein kinase-mediated endothelial cell death by hydrogen peroxide.Eur J Pharmacol. 2004 Feb 6;485(1-3):127-35. doi: 10.1016/j.ejphar.2003.11.079. Eur J Pharmacol. 2004. PMID: 14757132
-
Ebselen inhibits tumor necrosis factor-alpha-induced c-Jun N-terminal kinase activation and adhesion molecule expression in endothelial cells.Exp Cell Res. 2004 Jan 1;292(1):1-10. doi: 10.1016/j.yexcr.2003.08.003. Exp Cell Res. 2004. PMID: 14720501
-
Ebselen inhibits NO-induced apoptosis of differentiated PC12 cells via inhibition of ASK1-p38 MAPK-p53 and JNK signaling and activation of p44/42 MAPK and Bcl-2.J Neurochem. 2003 Dec;87(6):1345-53. doi: 10.1046/j.1471-4159.2003.02096.x. J Neurochem. 2003. PMID: 14713291
-
Intervention by retinoic acid in oxidative stress-induced apoptosis.Nephrol Dial Transplant. 2002;17 Suppl 9:84-7. doi: 10.1093/ndt/17.suppl_9.84. Nephrol Dial Transplant. 2002. PMID: 12386300 Review.
-
Ebselen, a selenoorganic compound as glutathione peroxidase mimic.Free Radic Biol Med. 1993 Mar;14(3):313-23. doi: 10.1016/0891-5849(93)90028-s. Free Radic Biol Med. 1993. PMID: 8458589 Review.
Cited by
-
Ebselen Is a Potential Anti-Osteoporosis Agent by Suppressing Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Osteoclast Differentiation In vitro and Lipopolysaccharide-Induced Inflammatory Bone Destruction In vivo.Int J Biol Sci. 2016 Feb 18;12(5):478-88. doi: 10.7150/ijbs.13815. eCollection 2016. Int J Biol Sci. 2016. PMID: 27019631 Free PMC article.
-
Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity.J Exp Neurosci. 2016 Sep 4;10(Suppl 1):23-48. doi: 10.4137/JEN.S39887. eCollection 2016. J Exp Neurosci. 2016. PMID: 27625575 Free PMC article. Review.
-
The adaptor protein SH2B1β reduces hydrogen peroxide-induced cell death in PC12 cells and hippocampal neurons.J Mol Signal. 2010 Sep 27;5:17. doi: 10.1186/1750-2187-5-17. J Mol Signal. 2010. PMID: 20868529 Free PMC article.
-
Promising therapeutic mechanism for Chinese herbal medicine in ameliorating renal fibrosis in diabetic nephropathy.Front Endocrinol (Lausanne). 2023 Jul 14;14:932649. doi: 10.3389/fendo.2023.932649. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37522131 Free PMC article. Review.
-
A polytherapy approach demonstrates therapeutic efficacy for the treatment of SOD1 associated amyotrophic lateral sclerosis.EBioMedicine. 2025 May;115:105692. doi: 10.1016/j.ebiom.2025.105692. Epub 2025 Apr 12. EBioMedicine. 2025. PMID: 40222103 Free PMC article.
References
-
- ABE J., BERK B.C. Reactive oxygen species as mediators of signal transduction in cardiovascular disease. Trends Cardiovasc. Med. 1998;8:59–64. - PubMed
-
- ANGEL P., KARIN M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129–157. - PubMed
-
- BHAT N.R., ZHANG P. Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J. Neurochem. 1999;72:112–119. - PubMed
-
- CHAN P.H. Oxygen radicals in focal cerebral ischemia. Brain Pathol. 1994;4:59–65. - PubMed
-
- CHAN P.H. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

