Differential coupling of 5-HT(1) receptors to G proteins of the G(i) family
- PMID: 12145108
- PMCID: PMC1573437
- DOI: 10.1038/sj.bjp.0704809
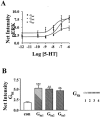
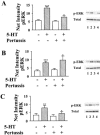
Differential coupling of 5-HT(1) receptors to G proteins of the G(i) family
Abstract
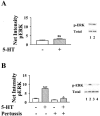
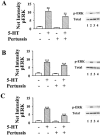
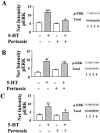
1: Since all 5-HT(1) receptors couple to G(i)-type G proteins and inhibit adenylyl cyclase, the functional significance of five distinct subtypes of 5-HT(1) receptors has been unclear. 2: In previous studies we have used transfected cells to demonstrate that 5-HT(1B) receptors can couple more efficiently than 5-HT(1A) receptors to activation of extracellular signal-regulated kinase (ERK) and to inhibition of adenylyl cyclase. These findings suggested the possibility that individual 5-HT(1) receptors differentially couple to isoforms of G(ialpha). 3: In the present study we utilized a model system in which pertussis toxin resistant forms of human G(ialpha1), G(ialpha2), and G(ialpha3) were used to directly compare the coupling of human 5-HT(1A), 5-HT(1B), and 5-HT(1D) receptors to each G(ialpha) in transfected human HeLa cells. 4: 5-HT(1A) receptors displayed a preference for G(ialpha1) and G(ialpha2), relative to G(ialpha3). Pertussis toxin resistant forms of G(ialpha1), G(ialpha2), and G(ialpha3) rescued 73%, 76%, and 44%, respectively, of the ERK activation stimulated by 5-HT in the absence of pertussis toxin. 5: In contrast, pertussis toxin resistant forms of G(ialpha1), G(ialpha2), and G(ialpha3) rescued 32%, 118%, and 35% of 5-HT(1B) receptor-stimulated activity, respectively, indicating that 5-HT(1B) receptors coupled primarily through G(ialpha2). A similar preference for G(ialpha2) was found in studies of the 5-HT(1D) receptor, where toxin resistant G(ialpha1), G(ialpha2), and G(ialpha3) rescued 30%, 70%, and 40% of activity, respectively. 6: In conclusion, the observed differential coupling of 5-HT(1) receptors to isoforms of G(ialpha), provides additional evidence for our previous findings that the subtypes of 5-HT(1) receptors exhibit similar, but distinct, functions.
Figures

Similar articles
-
Differential coupling of serotonin 5-HT1A and 5-HT1B receptors to activation of ERK2 and inhibition of adenylyl cyclase in transfected CHO cells.J Neurochem. 1999 Jul;73(1):162-8. doi: 10.1046/j.1471-4159.1999.0730162.x. J Neurochem. 1999. PMID: 10386967
-
Coupling of canine serotonin 5-HT(1B) and 5-HT(1D) receptor subtypes to the formation of inositol phosphates by dual interactions with endogenous G(i/o) and recombinant G(alpha15) proteins.J Neurochem. 2000 Sep;75(3):1180-9. doi: 10.1046/j.1471-4159.2000.0751180.x. J Neurochem. 2000. PMID: 10936201
-
Regulation of G protein activation and effector modulation by fusion proteins between the human 5-hydroxytryptamine(1A) receptor and the alpha subunit of G(i1): differences in receptor-constitutive activity imparted by single amino acid substitutions in G(i1)alpha.Mol Pharmacol. 1999 Oct;56(4):684-92. Mol Pharmacol. 1999. PMID: 10496950
-
Regulation of 5-HT(1A) and 5-HT(1B) receptor systems by phospholipid signaling cascades.Brain Res Bull. 2001 Nov 15;56(5):471-7. doi: 10.1016/s0361-9230(01)00645-1. Brain Res Bull. 2001. PMID: 11750792 Review.
-
Receptor signaling and structure: insights from serotonin-1 receptors.Trends Endocrinol Metab. 2001 Dec;12(10):453-60. doi: 10.1016/s1043-2760(01)00498-2. Trends Endocrinol Metab. 2001. PMID: 11701344 Review.
Cited by
-
Inflammation induces developmentally regulated sumatriptan inhibition of spinal synaptic transmission.Br J Pharmacol. 2020 Aug;177(16):3730-3743. doi: 10.1111/bph.15089. Epub 2020 Jul 8. Br J Pharmacol. 2020. PMID: 32352556 Free PMC article.
-
Serotonin and CGRP in migraine.Ann Neurosci. 2012 Apr;19(2):88-94. doi: 10.5214/ans.0972.7531.12190210. Ann Neurosci. 2012. PMID: 25205974 Free PMC article. Review.
-
Captive ERVWE1 triggers impairment of 5-HT neuronal plasticity in the first-episode schizophrenia by post-transcriptional activation of HTR1B in ALKBH5-m6A dependent epigenetic mechanisms.Cell Biosci. 2023 Nov 21;13(1):213. doi: 10.1186/s13578-023-01167-4. Cell Biosci. 2023. PMID: 37990254 Free PMC article.
-
The selective 5-HT1A receptor biased agonists, F15599 and F13714, show antidepressant-like properties after a single administration in the mouse model of unpredictable chronic mild stress.Psychopharmacology (Berl). 2021 Aug;238(8):2249-2260. doi: 10.1007/s00213-021-05849-0. Epub 2021 May 10. Psychopharmacology (Berl). 2021. PMID: 33973045 Free PMC article.
-
Development of 2-Aminotetralin-Type Serotonin 5-HT1 Agonists: Molecular Determinants for Selective Binding and Signaling at 5-HT1A, 5-HT1B, 5-HT1D, and 5-HT1F Receptors.ACS Chem Neurosci. 2024 Jan 17;15(2):357-370. doi: 10.1021/acschemneuro.3c00658. Epub 2023 Dec 27. ACS Chem Neurosci. 2024. PMID: 38150333 Free PMC article.
References
-
- AVISSAR S., NECHAMKIN Y., ROITMAN G., SCHREIBER G. Reduced G protein functions and immunoreactive levels in mononuclear leukocytes of patients with depression. Am. J. Psychiatry. 1997;154:211–217. - PubMed
-
- BRYS R., JOSSON K., CASTELLI M.P., JURZAK M., LIJNEN P., GOMMEREN W., LEYSEN J.E. Reconstitution of the human 5-HT1D receptor-G-protein coupling: evidence for constitutive activity and multiple receptor conformations. Mol. Pharmacol. 2000;57:1132–1141. - PubMed
-
- BUTKERAIT P., ZHENG Y., HALLAK H., GRAHAM T.E., MILLER H.A., BURRIS K.D., MOLINOFF P.B., MANNING D.R. Expression of the human 5-hydroxytryptamine1A receptor in Sf9 cells. J. Biol. Chem. 1995;270:18691–18699. - PubMed
-
- CLAWGES H.M., DEPREE K.M., PARKER E.M., GRABER S.G. Human 5-HT1 receptor subtypes exhibit distinct G protein coupling behaviors in membranes from Sf9 cells. Biochem. 1997;36:12930–12938. - PubMed
-
- COBB M., GOLDSMITH E. How MAP kinases are regulated. J. Biol. Chem. 1995;270:14843–14846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

