Early restriction of peripheral and proximal cell lineages during formation of the lung
- PMID: 12145322
- PMCID: PMC124949
- DOI: 10.1073/pnas.152238499
Early restriction of peripheral and proximal cell lineages during formation of the lung
Abstract
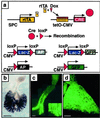
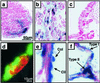
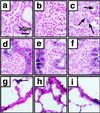
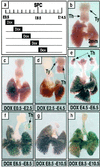
To establish the timing of lineage restriction among endodermal derivatives, we developed a method to label permanently subsets of lung precursor cells at defined times during development by using Cre recombinase to activate floxed alkaline phosphatase or green fluorescent protein genes under control of doxycycline-dependent surfactant protein C promoter. Extensive or complete labeling of peripheral lung, thyroid, and thymic epithelia, but not trachea, bronchi, or gastrointestinal tract occurred when mice were exposed to doxycycline from embryonic day (E) 4.5 to E6.5. Nonoverlapping cell lineages of conducting airways (trachea and bronchi), as distinct from those of peripheral airways (bronchioles, acini, and alveoli), were established well before formation of the definitive lung buds at E9-9.5. At E11.5, the labeled precursors of peripheral lung were restricted to relatively few cells along the bronchial tubes and clusters in bronchial tips and lateral buds. Thereafter, these cells underwent marked expansion to form the entire gas-exchange region in the lung. This study demonstrates early restriction of endodermal progenitor cells forming peripheral as compared with proximal airways, identifies distinct cell lineages in conducting airways, and distinguishes neuroepithelial and tracheal-bronchial gland cell lineages from those lining peripheral regions of the lung. This system for conditional gene addition or deletion is useful for the study of lung morphogenesis and gene function in vivo, and identifies progenitor cells that may serve as useful targets for cell or gene replacement for pulmonary disorders.
Figures


References
-
- Wells J. M. & Melton, D. A. (1999) Annu. Rev. Cell. Dev. Biol. 15, 393-410. - PubMed
-
- Wells J. M. & Melton, D. A. (2000) Development (Cambridge, U.K.) 127, 1563-1572. - PubMed
-
- Lawson K. A., Meneses, J. J. & Pedersen, R. A. (1986) Dev. Biol. 115, 325-339. - PubMed
-
- Perl A.-K. T. & Whitsett, J. A. (1999) Clin. Genet. 56, 14-27. - PubMed
-
- Warburton D., Schwarz, M., Tefft, D., Flores-Delgado, G., Anderson, K. D. & Cardoso, W. V. (2000) Mech. Dev. 92, 55-81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

