Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer's disease
- PMID: 12145324
- PMCID: PMC125060
- DOI: 10.1073/pnas.162228299
Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer's disease
Abstract
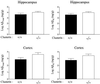
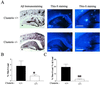
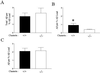
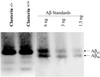
Studies have shown that clusterin (also called apolipoprotein J) can influence the structure and toxicity of amyloid-beta (Abeta) in vitro. To determine whether endogenous clusterin plays a role in influencing Abeta deposition, structure, and toxicity in vivo, we bred PDAPP mice, a transgenic mouse model of Alzheimer's disease, to clusterin(-/-) mice. By 12 months of age, PDAPP, clusterin(-/-) mice had similar levels of brain Abeta deposition as did PDAPP, clusterin(+/+) mice. Although Abeta deposition was similar, PDAPP, clusterin(-/-) mice had significantly fewer fibrillar Abeta (amyloid) deposits than PDAPP mice expressing clusterin. In the absence of clusterin, neuritic dystrophy associated with the deposited amyloid was markedly reduced, resulting in a dissociation between fibrillar amyloid formation and neuritic dystrophy. These findings demonstrate that clusterin markedly influences Abeta structure and neuritic toxicity in vivo and is likely to play an important role in Alzheimer's disease pathogenesis.
Figures

References
-
- Selkoe D. J. (2001) Physiol. Rev. 81, 741-766. - PubMed
-
- Van Nostrand W. E., Melchor, J. P., Cho, H. S., Greenberg, S. M. & Rebeck, G. W. (2001) J. Biol. Chem. 276, 32860-32866. - PubMed
-
- Nilsberth C., Westlind-Danielsson, A., Eckman, C. B., Condron, M. M., Axelman, K., Forsell, C., Stenh, C., Luthman, J., Teplow, D. B., Younkin, S. G., et al. (2001) Nat. Neurosci. 4, 887-893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

