Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects
- PMID: 12145326
- PMCID: PMC124907
- DOI: 10.1073/pnas.162352599
Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects
Abstract
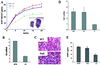
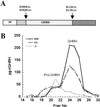
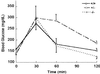
The subtilisin-like proprotein convertases PC1/3 (SPC3) and PC2 (SPC2) are believed to be the major endoproteolytic processing enzymes of the regulated secretory pathway. They are expressed together or separately in neuroendocrine cells throughout the brain and dispersed endocrine system in both vertebrates and invertebrates. Disruption of the gene-encoding mouse PC1/3 has now been accomplished and results in a syndrome of severe postnatal growth impairment and multiple defects in processing many hormone precursors, including hypothalamic growth hormone-releasing hormone (GHRH), pituitary proopiomelanocortin to adrenocorticotropic hormone, islet proinsulin to insulin and intestinal proglucagon to glucagon-like peptide-1 and -2. Mice lacking PC1/3 are normal at birth, but fail to grow normally and are about 60% of normal size at 10 weeks. They lack mature GHRH, have low pituitary growth hormone (GH) and hepatic insulin-like growth factor-1 mRNA levels and resemble phenotypically the "little" mouse (Gaylinn, B. D., Dealmeida, V. I., Lyons, C. E., Jr., Wu, K. C., Mayo, K. E. & Thorner, M. O. (1999) Endocrinology 140, 5066-5074) that has a mutant GHRH receptor. Despite a severe defect in pituitary proopiomelanocortin processing to mature adrenocorticotropic hormone, blood corticosterone levels are essentially normal. There is marked hyperproinsulinemia but without impairment of glucose tolerance. In contrast, PC2-null mice lack mature glucagon and are chronically hypoglycemic (Furuta, M., Yano, H., Zhou, A., Rouille, Y., Holst, J., Carroll, R., Ravazzola, M., Orci, L., Furuta, H. & Steiner, D. (1997) Proc. Natl. Acad. Sci. USA 94, 6646-6651). The PC1/3-null mice differ from a human subject reported with compound heterozygosity for defects in this gene, who was of normal stature but markedly obese from early life. The PC1/3-null mice are not obese. The basis for these phenotypic differences is an interesting topic for further study. These findings prove the importance of PC1/3 as a key neuroendocrine convertase.
Figures
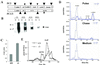
Similar articles
-
Impaired intestinal proglucagon processing in mice lacking prohormone convertase 1.Endocrinology. 2004 Mar;145(3):1349-55. doi: 10.1210/en.2003-0801. Epub 2003 Nov 20. Endocrinology. 2004. PMID: 14630721
-
Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide.J Biol Chem. 1995 Nov 3;270(44):26488-96. doi: 10.1074/jbc.270.44.26488. J Biol Chem. 1995. PMID: 7592866
-
Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2.J Biol Chem. 1998 Feb 6;273(6):3431-7. doi: 10.1074/jbc.273.6.3431. J Biol Chem. 1998. PMID: 9452465
-
The subtilisin/kexin family of precursor convertases. Emphasis on PC1, PC2/7B2, POMC and the novel enzyme SKI-1.Ann N Y Acad Sci. 1999 Oct 20;885:57-74. doi: 10.1111/j.1749-6632.1999.tb08665.x. Ann N Y Acad Sci. 1999. PMID: 10816641 Review.
-
The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals.Diabetes Metab. 1996 Apr;22(2):94-104. Diabetes Metab. 1996. PMID: 8792089 Review.
Cited by
-
Congenital proprotein convertase 1/3 deficiency causes malabsorptive diarrhea and other endocrinopathies in a pediatric cohort.Gastroenterology. 2013 Jul;145(1):138-148. doi: 10.1053/j.gastro.2013.03.048. Epub 2013 Apr 2. Gastroenterology. 2013. PMID: 23562752 Free PMC article.
-
The multifaceted proprotein convertases: their unique, redundant, complementary, and opposite functions.J Biol Chem. 2013 Jul 26;288(30):21473-81. doi: 10.1074/jbc.R113.481549. Epub 2013 Jun 17. J Biol Chem. 2013. PMID: 23775089 Free PMC article. Review.
-
Allelic clustering and ancestry-dependent frequencies of rs6232, rs6234, and rs6235 PCSK1 SNPs in a Northern Ontario population sample.J Community Genet. 2012 Oct;3(4):319-22. doi: 10.1007/s12687-012-0081-5. Epub 2012 Feb 4. J Community Genet. 2012. PMID: 22307923 Free PMC article.
-
Functional consequences of a novel variant of PCSK1.PLoS One. 2013;8(1):e55065. doi: 10.1371/journal.pone.0055065. Epub 2013 Jan 28. PLoS One. 2013. PMID: 23383060 Free PMC article.
-
Mice lacking PC1/3 expression in POMC-expressing cells do not develop obesity.Endocrinology. 2021 Mar 10;162(6):bqab055. doi: 10.1210/endocr/bqab055. Online ahead of print. Endocrinology. 2021. PMID: 33693631 Free PMC article.
References
-
- Zhou A., Webb, G., Zhu, X. & Steiner, D. F. (1999) J. Biol. Chem. 274, 20745-20748. - PubMed
-
- Muller L. & Lindberg, I., (2000) Progress in Nucleic Acid Research and Molecular Biology (Academic, New York), Vol. 63, pp. 69–108. - PubMed
-
- Rouillé Y., Martin, S. & Steiner, D. (1995) J. Biol. Chem. 270, 26488-26496. - PubMed
-
- Rouillé Y., Kantengwa, S., Irminger, J.-C. & Halban, P. A. (1997) J. Biol. Chem. 272, 32810-32816. - PubMed
-
- Mains R. E. & Eipper, B. A. (2000) in Handbook of Physiology, ed. McEwen, B. S. (Oxford Univ. Press, London), Vol. IV, pp. 85–101.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

