Neuregulin 1 and susceptibility to schizophrenia
- PMID: 12145742
- PMCID: PMC378543
- DOI: 10.1086/342734
Neuregulin 1 and susceptibility to schizophrenia
Abstract
The cause of schizophrenia is unknown, but it has a significant genetic component. Pharmacologic studies, studies of gene expression in man, and studies of mouse mutants suggest involvement of glutamate and dopamine neurotransmitter systems. However, so far, strong association has not been found between schizophrenia and variants of the genes encoding components of these systems. Here, we report the results of a genomewide scan of schizophrenia families in Iceland; these results support previous work, done in five populations, showing that schizophrenia maps to chromosome 8p. Extensive fine-mapping of the 8p locus and haplotype-association analysis, supplemented by a transmission/disequilibrium test, identifies neuregulin 1 (NRG1) as a candidate gene for schizophrenia. NRG1 is expressed at central nervous system synapses and has a clear role in the expression and activation of neurotransmitter receptors, including glutamate receptors. Mutant mice heterozygous for either NRG1 or its receptor, ErbB4, show a behavioral phenotype that overlaps with mouse models for schizophrenia. Furthermore, NRG1 hypomorphs have fewer functional NMDA receptors than wild-type mice. We also demonstrate that the behavioral phenotypes of the NRG1 hypomorphs are partially reversible with clozapine, an atypical antipsychotic drug used to treat schizophrenia.
Figures

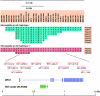


References
Electronic-Database Information
-
- Children's Hospital Oakland, BACPAC Resources, http://www.chori.org/bacpac/ (for RCPI-11 human BAC library)
-
- deCODE Genetics, http://www.decode.com/nrg1/markers/ (for SNPs and microsatellite markers in the NRG1 locus sequence)
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for NRG1 [AF491780 and TPA BK000383])
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for NRG1 [MIM 142445], schizophrenia [MIM 181500], and SCZD6 [MIM 603013])
-
- Phred/Phrap/Consed home page, http://bozeman.mbt.washington.edu/index.html
References
-
- Bilder RM, Corcoran R, Frith CD (1996) Neuropsychology and neurophysiology in schizophrenia. Curr Opin Psychiatry 9:57–62
-
- Blouin JL, Dombroski BA, Nath SK, Lasseter VK, Wolyniec PS, Nestadt G, Thornquist M, et al (1998) Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nat Genet 20:70–73 - PubMed
-
- Braff DL, Geyer MA (1990) Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry 47:181–188 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

