Ability of nonpathogenic Fusarium oxysporum strain Fo47 to induce resistance against Pythium ultimum infection in cucumber
- PMID: 12147506
- PMCID: PMC124014
- DOI: 10.1128/AEM.68.8.4044-4060.2002
Ability of nonpathogenic Fusarium oxysporum strain Fo47 to induce resistance against Pythium ultimum infection in cucumber
Abstract
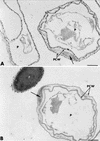
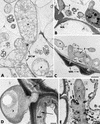
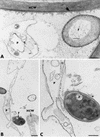
The influence exerted by nonpathogenic Fusarium oxysporum strain Fo47 in triggering cucumber protection against infection by Pythium ultimum was investigated ultrastructurally. Macroscopic and microscopic observations of the pathogen colony in dual cultures revealed that reduction of Pythium growth was associated with marked disorders, including generalized disorganization of the host cytoplasm, retraction of the plasmalemma, and complete loss of the protoplasm. Cytochemical labeling of cellulose with an exoglucanase-gold complex showed that the cellulose component of the host cell walls was structurally preserved at a time when the host cytoplasm had undergone complete disorganization. A similar antagonistic process was observed at the root cell surface. Most striking and interesting was the finding that mycoparasitism, as evidenced by the frequent occurrence of Fo47 hyphae within nearly empty cells of the pathogen, occurred not only at the root surface but also within the invaded root tissues. The specific labeling pattern obtained with the exoglucanase-gold complex confirmed that Fo47 successfully penetrated cells of the pathogen, both in the rhizosphere and inside the root tissues. Pythium cells that could evade the first defensive line in the rhizosphere could penetrate the root epidermis, but their growth was restricted to the outermost tissues. Positive correlations between Fo47 treatment and induced resistance to infection by P. ultimum in cucumber were confirmed by (i) the reduction of pathogen viability; (ii) the elaboration of newly formed barriers, a phenomenon which was not seen in Fo47-free plants, where the pathogen proliferated in all root tissues within a few days; and (iii) the occlusion of intercellular spaces with a dense material likely enriched in phenolics. Taken together, our observations provide the first convincing evidence that Fo47 exerts a direct inhibitory effect on P. ultimum through a combination of antibiosis and mycoparasitism, in addition to being a strong inducer of plant defense reactions.
Figures








Similar articles
-
Biocontrol by Fusarium oxysporum Using Endophyte-Mediated Resistance.Front Plant Sci. 2020 Feb 6;11:37. doi: 10.3389/fpls.2020.00037. eCollection 2020. Front Plant Sci. 2020. PMID: 32117376 Free PMC article. Review.
-
Treatment with the Mycoparasite Pythium oligandrum Triggers Induction of Defense-Related Reactions in Tomato Roots When Challenged with Fusarium oxysporum f. sp. radicis-lycopersici.Phytopathology. 1997 Jan;87(1):108-22. doi: 10.1094/PHYTO.1997.87.1.108. Phytopathology. 1997. PMID: 18945162
-
Chitinase and beta-1,3-glucanase enzyme production by the mycoparasite Clonostachys rosea f. catenulata against fungal plant pathogens.Can J Microbiol. 2009 Apr;55(4):356-67. doi: 10.1139/w08-156. Can J Microbiol. 2009. PMID: 19396235
-
Bacterial-Mediated Induced Resistance in Cucumber: Beneficial Effect of the Endophytic Bacterium Serratia plymuthica on the Protection Against Infection by Pythium ultimum.Phytopathology. 2000 Jan;90(1):45-56. doi: 10.1094/PHYTO.2000.90.1.45. Phytopathology. 2000. PMID: 18944571
-
Effects on growth and comparison of root tissue colonization patterns of Eucalyptus viminalis by pathogenic and nonpathogenic strains of Fusarium oxysporum.New Phytol. 2000 May;146(2):317-324. doi: 10.1046/j.1469-8137.2000.00629.x. New Phytol. 2000. PMID: 33862965 Review.
Cited by
-
Metatranscriptomic Comparison of Endophytic and Pathogenic Fusarium-Arabidopsis Interactions Reveals Plant Transcriptional Plasticity.Mol Plant Microbe Interact. 2021 Sep;34(9):1071-1083. doi: 10.1094/MPMI-03-21-0063-R. Epub 2021 Oct 11. Mol Plant Microbe Interact. 2021. PMID: 33856230 Free PMC article.
-
Diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of Witches' Broom Disease.Int J Biol Sci. 2005;1(1):24-33. doi: 10.7150/ijbs.1.24. Epub 2005 Feb 1. Int J Biol Sci. 2005. PMID: 15951847 Free PMC article.
-
Modeling competition for infection sites on roots by nonpathogenic strains of Fusarium oxysporum.Mycopathologia. 2007 Jan;163(1):9-20. doi: 10.1007/s11046-006-0080-3. Mycopathologia. 2007. PMID: 17216327
-
Biocontrol by Fusarium oxysporum Using Endophyte-Mediated Resistance.Front Plant Sci. 2020 Feb 6;11:37. doi: 10.3389/fpls.2020.00037. eCollection 2020. Front Plant Sci. 2020. PMID: 32117376 Free PMC article. Review.
-
Differential Colonization of the Plant Vasculature Between Endophytic Versus Pathogenic Fusarium oxysporum Strains.Mol Plant Microbe Interact. 2023 Jan;36(1):4-13. doi: 10.1094/MPMI-08-22-0166-SC. Epub 2023 Jan 9. Mol Plant Microbe Interact. 2023. PMID: 36279112 Free PMC article.
References
-
- Alabouvette, C. 1999. Fusarium wilt suppressive soils: an example of disease-suppressive soils. Aust. J. Plant Pathol. 28:57-64.
-
- Alabouvette, C., and Y. Couteaudier. 1992. Biological control of fusarium wilts with nonpathogenic fusaria, p. 415-426. In E. C. Tjamos, G. C. Papavizas, and R. Cook (ed.), Biological control of plant diseases: progress and challenges for the future. Plenum Press, New York, N.Y.
-
- Alabouvette, C., Y. Couteaudier, Y., and J. Louvet. 1985. Soils suppressive to fusarium wilt: mechanisms and management of suppressiveness, p. 101-106. In C. A. Parker, A. D. Rovira, K. J. Moore, P. T. W. Wong, and J. F. Kollmorgen (ed.), Ecology and management of soilborne plant pathogens. American Phytopathological Society, St. Paul, Minn.
-
- Alabouvette, C., D. De la Broise, P. Lemanceau, Y. Couteaudier, and J. Louvet. 1987. Utilisation de souches non pathogènes de Fusarium spp. pour lutter contre les fusarioses: situation actuelle dans la pratique. EPPO Bull. 17:665-674.
-
- Alabouvette, C., P. Lemanceau, and C. Steinberg. 1996. Biological control of Fusarium wilts: opportunities for developing a commercial product, p. 192-212. In R. Hall (ed.), Principles and practice of managing soilborne plant pathogens. American Phytopathological Press, St. Paul, Minn.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

