Detection of anatoxin-a(s) in environmental samples of cyanobacteria by using a biosensor with engineered acetylcholinesterases
- PMID: 12147513
- PMCID: PMC123992
- DOI: 10.1128/AEM.68.8.4102-4106.2002
Detection of anatoxin-a(s) in environmental samples of cyanobacteria by using a biosensor with engineered acetylcholinesterases
Abstract
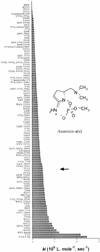
Bioassays are little used to detect individual toxins in the environment because, compared to analytical methods, these assays are still limited by several problems, such as the sensitivity and specificity of detection. We tentatively solved these two drawbacks for detection of anatoxin-a(s) by engineering an acetylcholinesterase to increase its sensitivity and by using a combination of mutants to obtain increased analyte specificity. Anatoxin-a(s), a neurotoxin produced by some freshwater cyanobacteria, was detected by measuring the inhibition of acetylcholinesterase activity. By using mutated enzyme, the sensitivity of detection was brought to below the nanomole-per-liter level. However, anatoxin-a(s) is an organophosphorous compound, as are several synthetic molecules which are widely used as insecticides. The mode of action of these compounds is via inhibition of acetylcholinesterase, which makes the biotest nonspecific. The use of a four-mutant set of acetylcholinesterase variants, two mutants that are sensitive to anatoxin-a(s) and two mutants that are sensitive to the insecticides, allows specific detection of the cyanobacterial neurotoxin.
Figures



Similar articles
-
A disposable acetylcholinesterase-based electrode biosensor to detect anatoxin-a(s) in water.Anal Bioanal Chem. 2002 Jan;372(2):322-6. doi: 10.1007/s00216-001-1127-4. Epub 2001 Dec 20. Anal Bioanal Chem. 2002. PMID: 11936106
-
Anatoxin-a in Irish freshwater and cyanobacteria, determined using a new fluorimetric liquid chromatographic method.Toxicon. 1997 Jun;35(6):963-71. doi: 10.1016/s0041-0101(96)00201-2. Toxicon. 1997. PMID: 9241789
-
Detection of an anatoxin-a(s)-like anticholinesterase in natural blooms and cultures of cyanobacteria/blue-green algae from Danish lakes and in the stomach contents of poisoned birds.Toxicon. 1997 Jun;35(6):901-13. doi: 10.1016/s0041-0101(96)00190-0. Toxicon. 1997. PMID: 9241784
-
Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a.Environ Int. 2007 Nov;33(8):1070-89. doi: 10.1016/j.envint.2007.06.003. Epub 2007 Jul 30. Environ Int. 2007. PMID: 17673293 Review.
-
Cyanobacterial toxins--occurrence, biosynthesis and impact on human affairs.Mol Nutr Food Res. 2006 Jan;50(1):7-17. doi: 10.1002/mnfr.200500162. Mol Nutr Food Res. 2006. PMID: 16304634 Review.
Cited by
-
Understanding the Risks of Diffusion of Cyanobacteria Toxins in Rivers, Lakes, and Potable Water.Toxins (Basel). 2023 Sep 20;15(9):582. doi: 10.3390/toxins15090582. Toxins (Basel). 2023. PMID: 37756009 Free PMC article. Review.
-
A Direct Analysis of β-N-methylamino-l-alanine Enantiomers and Isomers and Its Application to Cyanobacteria and Marine Mollusks.Toxins (Basel). 2023 Nov 1;15(11):639. doi: 10.3390/toxins15110639. Toxins (Basel). 2023. PMID: 37999501 Free PMC article.
-
Recent Trends in Biosensors for Environmental Quality Monitoring.Sensors (Basel). 2022 Feb 15;22(4):1513. doi: 10.3390/s22041513. Sensors (Basel). 2022. PMID: 35214408 Free PMC article. Review.
-
Immunoassays and biosensors for the detection of cyanobacterial toxins in water.Sensors (Basel). 2013 Nov 5;13(11):15085-112. doi: 10.3390/s131115085. Sensors (Basel). 2013. PMID: 24196435 Free PMC article. Review.
-
Addressing the Selectivity of Enzyme Biosensors: Solutions and Perspectives.Sensors (Basel). 2021 Apr 26;21(9):3038. doi: 10.3390/s21093038. Sensors (Basel). 2021. PMID: 33926034 Free PMC article. Review.
References
-
- Bachmann, T. T., and R. D. Schmid. 1999. A disposable multielectrode biosensor for the discrimination of organophosphate and carbonate insecticides in the submicromolar range using native and recombinant variants of acetylcholinesterase. Anal. Chim. Acta 401:95-103.
-
- Bachmann, T. T., B. Leca, F. Vilatte, J.-L. Marty, D. Fournier, and R. D. Schmid. 2000. Improved multianalyte detection of organophosphates and carbamates with disposable multielectrode biosensors using recombinant mutants of Drosophila acetylcholinesterase and artificial neural network. Biosens. Bioelect. 15:193-201. - PubMed
-
- Beasley, V. R., R. W. Coppock, J. Simon, R. Ely, W. B. Buck, and R. A. Corley. 1983. Apparent blue-green algae poisoning in swine subsequent to ingestion of a bloom dominated by Anabaena spiroides. J. Am. Vet. Assoc. 182:413-414. - PubMed
-
- Boublik, Y., P. Saint-Aguet, A. Lougarre, M. Arnaud, F. Villatte, S. Estrada-Mondaca, and D. Fournier. 2002. Acetylcholinesterase engineering for detection of insecticide residues. Protein Eng. 15:43-50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

