The ferroxidase activity of yeast frataxin
- PMID: 12149269
- PMCID: PMC4526241
- DOI: 10.1074/jbc.M206711200
The ferroxidase activity of yeast frataxin
Abstract
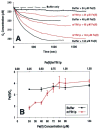
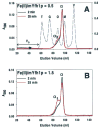
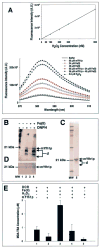
Frataxin is required for maintenance of normal mitochondrial iron levels and respiration. The mature form of yeast frataxin (mYfh1p) assembles stepwise into a multimer of 840 kDa (alpha(48)) that accumulates iron in a water-soluble form. Here, two distinct iron oxidation reactions are shown to take place during the initial assembly step (alpha --> alpha(3)). A ferroxidase reaction with a stoichiometry of 2 Fe(II)/O(2) is detected at Fe(II)/mYfh1p ratios of < or = 0.5. Ferroxidation is progressively overcome by autoxidation at Fe(II)/mYfh1p ratios of >0.5. Gel filtration analysis indicates that an oligomer of mYfh1p, alpha(3), is responsible for both reactions. The observed 2 Fe(II)/O(2) stoichiometry implies production of H(2)O(2) during the ferroxidase reaction. However, only a fraction of the expected total H(2)O(2) is detected in solution. Oxidative degradation of mYfh1p during the ferroxidase reaction suggests that most H(2)O(2) reacts with the protein. Accordingly, the addition of mYfh1p to a mixture of Fe(II) and H(2)O(2) results in significant attenuation of Fenton chemistry. Multimer assembly is fully inhibited under anaerobic conditions, indicating that mYfh1p is activated by Fe(II) in the presence of O(2). This combination induces oligomerization and mYfh1p-catalyzed Fe(II) oxidation, starting a process that ultimately leads to the sequestration of as many as 50 Fe(II)/subunit inside the multimer.
Figures


References
-
- Tangeras A. Biochim Biophys Acta. 1985;843:199–207. - PubMed
-
- Darnell J, Lodish H, Baltimore D. Molecular Cell Biology. Scientific American Books; New York: 1990. pp. 583–616.
-
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; Oxford, UK: 1999. pp. 1–34.
-
- Flatmark T, Romslo I. J Biol Chem. 1975;250:6433–6438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

