Effect of Na(+) flow on Cd(2+) block of tetrodotoxin-resistant Na(+) channels
- PMID: 12149278
- PMCID: PMC2234463
- DOI: 10.1085/jgp.20018536
Effect of Na(+) flow on Cd(2+) block of tetrodotoxin-resistant Na(+) channels
Abstract
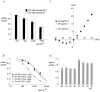
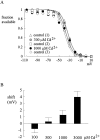
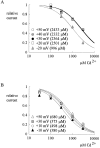
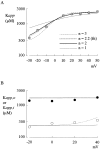
Tetrodotoxin-resistant (TTX-R) Na(+) channels are 1,000-fold less sensitive to TTX than TTX-sensitive (TTX-S) Na(+) channels. On the other hand, TTX-R channels are much more susceptible to external Cd(2+) block than TTX-S channels. A cysteine (or serine) residue situated just next to the aspartate residue of the presumable selectivity filter "DEKA" ring of the TTX-R channel has been identified as the key ligand determining the binding affinity of both TTX and Cd(2+). In this study we demonstrate that the binding affinity of Cd(2+) to the TTX-R channels in neurons from dorsal root ganglia has little intrinsic voltage dependence, but is significantly influenced by the direction of Na(+) current flow. In the presence of inward Na(+) current, the apparent dissociation constant of Cd(2+) ( approximately 200 microM) is approximately 9 times smaller than that in the presence of outward Na(+) current. The Na(+) flow-dependent binding affinity change of Cd(2+) block is true no matter whether the direction of Na(+) current is secured by asymmetrical chemical gradient (e.g., 150 mM Na(+) vs. 150 mM Cs(+) on different sides of the membrane, 0 mV) or by asymmetrical electrical gradient (e.g., 150 mM Na(+) on both sides of the membrane, -20 mV vs. 20 mV). These findings suggest that Cd(2+) is a pore blocker of TTX-R channels with its binding site located in a multiion, single-file region near the external pore mouth. Quantitative analysis of the flow dependence with the flux-coupling equation reveals that at least two Na(+) ions coexist with the blocking Cd(2+) ion in this pore region in the presence of 150 mM ambient Na(+). Thus, the selectivity filter of the TTX-R Na(+) channels in dorsal root ganglion neurons might be located in or close to a multiion single-file pore segment connected externally to a wide vestibule, a molecular feature probably shared by other voltage-gated cationic channels, such as some Ca(2+) and K(+) channels.
Figures





Similar articles
-
Block of tetrodotoxin-resistant Na+ channel pore by multivalent cations: gating modification and Na+ flow dependence.J Gen Physiol. 2004 Jul;124(1):27-42. doi: 10.1085/jgp.200409054. J Gen Physiol. 2004. PMID: 15226363 Free PMC article.
-
Expression and kinetic properties of Na(+) currents in rat cardiac dorsal root ganglion neurons.Brain Res. 2002 Aug 23;947(1):67-77. doi: 10.1016/s0006-8993(02)02908-6. Brain Res. 2002. PMID: 12144854
-
Characteristics of ropivacaine block of Na+ channels in rat dorsal root ganglion neurons.Anesth Analg. 2000 Nov;91(5):1213-20. doi: 10.1097/00000539-200011000-00031. Anesth Analg. 2000. PMID: 11049911
-
Molecular pore structure of voltage-gated sodium and calcium channels.Braz J Med Biol Res. 1994 Dec;27(12):2781-802. Braz J Med Biol Res. 1994. PMID: 7550000 Review.
-
Tetrodotoxin-resistant sodium channels.Cell Mol Neurobiol. 1994 Jun;14(3):227-44. doi: 10.1007/BF02088322. Cell Mol Neurobiol. 1994. PMID: 7712513 Free PMC article. Review.
Cited by
-
TMEM16C facilitates Na(+)-activated K+ currents in rat sensory neurons and regulates pain processing.Nat Neurosci. 2013 Sep;16(9):1284-90. doi: 10.1038/nn.3468. Epub 2013 Jul 21. Nat Neurosci. 2013. PMID: 23872594 Free PMC article.
-
Block of tetrodotoxin-resistant Na+ channel pore by multivalent cations: gating modification and Na+ flow dependence.J Gen Physiol. 2004 Jul;124(1):27-42. doi: 10.1085/jgp.200409054. J Gen Physiol. 2004. PMID: 15226363 Free PMC article.
-
Inhibition of tetrodotoxin-resistant sodium current in dorsal root ganglia neurons mediated by D1/D5 dopamine receptors.Mol Pain. 2013 Nov 28;9:60. doi: 10.1186/1744-8069-9-60. Mol Pain. 2013. PMID: 24283218 Free PMC article.
-
Voltage-dependent inhibition of rat skeletal muscle sodium channels by aminoglycoside antibiotics.Pflugers Arch. 2004 May;448(2):204-13. doi: 10.1007/s00424-004-1244-y. Epub 2004 Feb 13. Pflugers Arch. 2004. PMID: 14963710
-
Ionic flow enhances low-affinity binding: a revised mechanistic view into Mg2+ block of NMDA receptors.J Physiol. 2010 Feb 15;588(Pt 4):633-50. doi: 10.1113/jphysiol.2009.178913. Epub 2009 Dec 21. J Physiol. 2010. PMID: 20026615 Free PMC article.
References
-
- Akopian, A.N., L. Sivilotti, and J.N. Wood. 1996. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 379:257–262. - PubMed
-
- Akopian, A.N., V. Souslova, S. England, K. Okuse, N. Ogata, J. Ure, A. Smith, B.J. Kerr, S.B. McMahon, S. Boyce, et al. 1999. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 2:541–548. - PubMed
-
- Backx, P.H., D.T. Yue, J.H. Lawrence, E. Marban, and G.F. Tomaselli. 1992. Molecular localization of an ion binding site within the pore of mammalian sodium channels. Science. 257:248–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

