Elimination of the BK(Ca) channel's high-affinity Ca(2+) sensitivity
- PMID: 12149279
- PMCID: PMC2234461
- DOI: 10.1085/jgp.20028627
Elimination of the BK(Ca) channel's high-affinity Ca(2+) sensitivity
Abstract
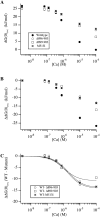
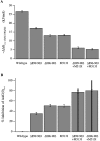
We report here a combination of site-directed mutations that eliminate the high-affinity Ca(2+) response of the large-conductance Ca(2+)-activated K(+) channel (BK(Ca)), leaving only a low-affinity response blocked by high concentrations of Mg(2+). Mutations at two sites are required, the "Ca(2+) bowl," which has been implicated previously in Ca(2+) binding, and M513, at the end of the channel's seventh hydrophobic segment. Energetic analyses of mutations at these positions, alone and in combination, argue that the BK(Ca) channel contains three types of Ca(2+) binding sites, one of low affinity that is Mg(2+) sensitive (as has been suggested previously) and two of higher affinity that have similar binding characteristics and contribute approximately equally to the power of Ca(2+) to influence channel opening. Estimates of the binding characteristics of the BK(Ca) channel's high-affinity Ca(2+)-binding sites are provided.
Figures





References
-
- Adelman, J.P., K.Z. Shen, M.P. Kavanaugh, R.A. Warren, Y.N. Wu, A. Lagrutta, C.T. Bond, and R.A. North. 1992. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 9:209–216. - PubMed
-
- Bers, D., C. Patton, and R. Nuccitelli. 1994. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 40:3–29. - PubMed
-
- Branden, C., and J. Tooze. 1991. Enzymes that bind nucleotides. In Introduction to Protein Structure. New York, Garland Publishing. 141–159.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

