Effects of aging on central and peripheral mammalian clocks
- PMID: 12149444
- PMCID: PMC125050
- DOI: 10.1073/pnas.152318499
Effects of aging on central and peripheral mammalian clocks
Abstract
Circadian organization changes with age, but we do not know the extent to which age-related changes are the result of alterations in the central pacemakers, the peripheral oscillators, or the coupling mechanisms that hold the system together. By using transgenic rats with a luciferase (luc) reporter, we assessed the effects of aging on the rhythm of expression of the Period 1 (Per1) gene in the suprachiasmatic nucleus (SCN) and in peripheral tissues. Young (2 months) and aged (24-26 months) Per1-luc transgenic rats, entrained to light-dark cycles, were killed, and tissues were removed and cultured. Per1-luc expression was measured from 10 tissues. In the SCN, the central mammalian pacemaker, Per1-luc expression was robustly rhythmic for more than 7 weeks in culture. The only difference between SCN rhythmicity in young and old rats was a small but significant age-related shortening of the free-running period. Circadian rhythmicity in some peripheral tissues was unaffected by aging, whereas rhythmicity in other tissues was either phase advanced relative to the light cycle or absent. Those tissues that were arrhythmic could be induced to oscillate by application of forskolin, suggesting that they retained the capacity to oscillate but were not being appropriately driven in vivo. Overall, the results provide new insights into the effects of aging on the mammalian circadian system. Aging seems to affect rhythms in some but not in all tissues and may act primarily on interactions among circadian oscillators, perhaps attenuating the ability of the SCN to drive damped oscillators in the periphery.
Figures
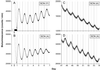
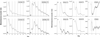
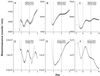
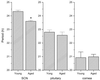

References
-
- Klein D. C., Moore, R. Y. & Reppert, S. M., (1991) The Mind's Clock (Oxford Univ. Press, New York).
-
- Moore R. Y. & Eichler, V. B. (1972) Brain Res. 42, 201-206. - PubMed
-
- Rusak B. & Zucker, I. (1979) Physiol. Rev. 59, 449-526. - PubMed
-
- Meyer-Bernstein E. L., Jetton, A. E., Matsumoto, S. I., Markuns, J. F., Lehman, M. N. & Bittman, E. L. (1999) Endocrinology 140, 207-218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

