De novo determination of peptide structure with solid-state magic-angle spinning NMR spectroscopy
- PMID: 12149447
- PMCID: PMC124901
- DOI: 10.1073/pnas.152346599
De novo determination of peptide structure with solid-state magic-angle spinning NMR spectroscopy
Abstract
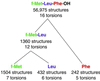
The three-dimensional structure of the chemotactic peptide N-formyl-l-Met-l-Leu-l-Phe-OH was determined by using solid-state NMR (SSNMR). The set of SSNMR data consisted of 16 (13)C-(15)N distances and 18 torsion angle constraints (on 10 angles), recorded from uniformly (13)C,(15)N- and (15)N-labeled samples. The peptide's structure was calculated by means of simulated annealing and a newly developed protocol that ensures that all of conformational space, consistent with the structural constraints, is searched completely. The result is a high-quality structure of a molecule that has thus far not been amenable to single-crystal diffraction studies. The extensions of the SSNMR techniques and computational methods to larger systems appear promising.
Figures

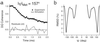


References
-
- Wüthrich K., (1986) NMR of Proteins and Nucleic Acids (Wiley, New York).
-
- Gayathri C., Bothner-By, A. A., van Zijl, P. C. M. & MacLean, C. (1982) Chem. Phys. Lett. 87, 192-196.
-
- Tjandra N. & Bax, A. (1997) Science 278, 1111-1114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

