Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression
- PMID: 12149448
- PMCID: PMC125023
- DOI: 10.1073/pnas.152597199
Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression
Abstract
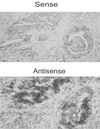
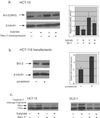
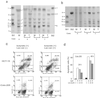
Human plasma membrane-associated sialidase (Neu3) is unique in specifically hydrolyzing gangliosides, thought to participate in cell differentiation and transmembrane signaling, thereby playing crucial roles in the regulation of cell surface functions. We have discovered levels of mRNA for this sialidase to be increased in restricted cases of human colon cancer by 3- to 100-fold compared with adjacent nontumor mucosa (n = 32), associated with significant elevation in sialidase activity in tumors (n = 50). In situ hybridization showed the sialidase expression in epithelial elements of adenocarcinomas. In cultured human colon cancer cells, the sialidase level was down-regulated in the process of differentiation and apoptosis induced by sodium butyrate, whereas lysosomal sialidase (Neu1) was up-regulated. Transfection of the sialidase gene into colon cancer cells inhibited apoptosis and was accompanied by increased Bcl-2 and decreased caspase expression. Colon cancer exhibited a marked accumulation of lactosylceramide, a possible sialidase product, and addition of the glycolipid to the culture reduced apoptotic cells during sodium butyrate treatment. These results indicate that high expression of the sialidase in cancer cells leads to protection against programmed cell death, probably modulation of gangliosides. This finding provides a possible sialidase target for diagnosis and therapy of colon cancer.
Figures



Comment in
-
Glycosylation defining cancer malignancy: new wine in an old bottle.Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10231-3. doi: 10.1073/pnas.172380699. Epub 2002 Jul 30. Proc Natl Acad Sci U S A. 2002. PMID: 12149519 Free PMC article. Review. No abstract available.
References
-
- Yogeeswaran G. & Salk, P. (1981) Science 212, 1514-1516. - PubMed
-
- Collard J. G., Schijven, J. F., Bikker, A., La Riviere, G., Bolscher, J. G. M. & Roos, E. (1986) Cancer Res. 46, 3521-3527. - PubMed
-
- Dennis J. W., Lafarté, S., Waghorne, C., Breitman, M. L. & Kerbel, R. S. (1987) Science 236, 582-585. - PubMed
-
- Passaniti A. & Hart, G. W. (1988) J. Biol. Chem. 263, 7591-7603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

