Tetracyclines affect prion infectivity
- PMID: 12149459
- PMCID: PMC125061
- DOI: 10.1073/pnas.162195499
Tetracyclines affect prion infectivity
Abstract
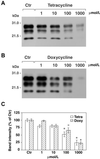
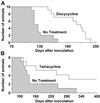
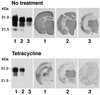
Prion diseases are transmissible neurodegenerative disorders of humans and animals for which no effective treatment is available. Conformationally altered, protease-resistant forms of the prion protein (PrP) termed PrP(Sc) are critical for disease transmissibility and pathogenesis, thus representing a primary target for therapeutic strategies. Based on previous findings that tetracyclines revert abnormal physicochemical properties and abolish neurotoxicity of PrP peptides in vitro, we tested the ability of these compounds to interact with PrP(Sc) from patients with the new variant of Creutzfeldt-Jakob disease (vCJD) and cattle with bovine spongiform encephalopathy (BSE). The incubation with tetracycline hydrochloride or doxycycline hyclate at concentrations ranging from 10 microM to 1 mM resulted in a dose-dependent decrease in protease resistance of PrP(Sc). This finding prompted us to investigate whether tetracyclines affect prion infectivity by using an animal model of disease. Syrian hamsters were injected intracerebrally with 263K scrapie-infected brain homogenate that was coincubated with 1 mM tetracycline hydrochloride, 1 mM doxycycline hyclate, or vehicle solution before inoculation. Hamsters injected with tetracycline-treated inoculum showed a significant delay in the onset of clinical signs of disease and prolonged survival time. These effects were paralleled by a delay in the appearance of magnetic-resonance abnormalities in the thalamus, neuropathological changes, and PrP(Sc) accumulation. When tetracycline was preincubated with highly diluted scrapie-infected inoculum, one third of hamsters did not develop disease. Our data suggest that these well characterized antibiotics reduce prion infectivity through a direct interaction with PrP(Sc) and are potentially useful for inactivation of BSE- or vCJD-contaminated products and prevention strategies.
Figures

References
-
- Prusiner S. B. (1998) Proc. Natl. Acad. Sci. USA 90, 10962-10966.
-
- DeArmond S. J. & Prusiner, S. B. (1997) in Greenfield's Neuropathology, eds. Graham, D. I. & Lantos, P. L. (Arnold, London), pp. 235–280.
-
- Tagliavini F., Forloni, G., D'Ursi, P., Bugiani, O. & Salmona, M. (2001) Adv. Protein Chem. 57, 171-202. - PubMed
-
- Kocisko D. A., Come, J. H., Priola, S. A., Chesebro, B., Raymond, G. J., Lansbury, P. T. & Caughey, B. (1994) Nature (London) 370, 471-474. - PubMed
-
- Saborio G. P., Permanne, B. & Soto, C. (2001) Nature (London) 411, 810-813. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

