Neuregulin-1 promotes formation of the murine cardiac conduction system
- PMID: 12149465
- PMCID: PMC124940
- DOI: 10.1073/pnas.162301699
Neuregulin-1 promotes formation of the murine cardiac conduction system
Abstract
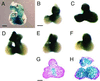
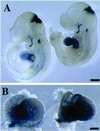
The cardiac conduction system is a network of cells responsible for the rhythmic and coordinated excitation of the heart. Components of the murine conduction system, including the peripheral Purkinje fibers, are morphologically indistinguishable from surrounding cardiomyocytes, and a paucity of molecular markers exists to identify these cells. The murine conduction system develops in close association with the endocardium. Using the recently identified CCS-lacZ line of reporter mice, in which lacZ expression delineates the embryonic and fully mature conduction system, we tested the ability of several endocardial-derived paracrine factors to convert contractile cardiomyocytes into conduction-system cells as measured by ectopic reporter gene expression in the heart. In this report we show that neuregulin-1, a growth and differentiation factor essential for ventricular trabeculation, is sufficient to induce ectopic expression of the lacZ conduction marker. This inductive effect of neuregulin-1 was restricted to a window of sensitivity between 8.5 and 10.5 days postcoitum. Using the whole mouse embryo culture system, neuregulin-1 was shown to regulate lacZ expression within the embryonic heart, whereas its expression in other tissues remained unaffected. We describe the electrical activation pattern of the 9.5-days postcoitum embryonic mouse heart and show that treatment with neuregulin-1 results in electrophysiological changes in the activation pattern consistent with a recruitment of cells to the conduction system. This study supports the hypothesis that endocardial-derived neuregulins may be the major endogenous ligands responsible for inducing murine embryonic cardiomyocytes to differentiate into cells of the conduction system.
Figures



References
-
- Gourdie R. G., Mima, T., Thompson, R. P. & Mikawa, T. (1995) Development (Cambridge, U.K.) 121, 1423-1431. - PubMed
-
- Cheng G., Litchenberg, W. H., Cole, G. J., Mikawa, T., Thompson, R. P. & Gourdie, R. G. (1999) Development (Cambridge, U.K.) 126, 5041-5049. - PubMed
-
- Mikawa T. & Fischman, D. A. (1996) Annu. Rev. Physiol. 58, 509-521. - PubMed
-
- Yoshizumi M., Kurihara, H., Sugiyama, T., Takaku, F., Yanagisawa, M., Masaki, T. & Yazaki, Y. (1989) Biochem. Biophys. Res. Commun. 161, 859-864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

