Absolute requirement of spermidine for growth and cell cycle progression of fission yeast (Schizosaccharomyces pombe)
- PMID: 12149471
- PMCID: PMC124914
- DOI: 10.1073/pnas.162362899
Absolute requirement of spermidine for growth and cell cycle progression of fission yeast (Schizosaccharomyces pombe)
Abstract
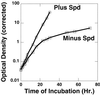
Schizosaccharomyces pombe cells that cannot synthesize spermidine or spermine because of a deletion-insertion in the gene coding for S-adenosylmethionine decarboxylase (Deltaspe2) have an absolute requirement for spermidine for growth. Flow cytometry studies show that in the absence of spermidine an overall delay of the cell cycle progression occurs with some accumulation of cells in the G(1) phase; as little as 10(-6) M spermidine is sufficient to maintain normal cell cycle distribution and normal growth. Morphologically some of the spermidine-deprived cells become spherical at an early stage with little evidence of cell division. On further incubation in the spermidine-deprived medium, growth occurs in most of the cells, not by cell division but rather by cell elongation, with an abnormal distribution of the actin cytoskeleton, DNA (4', 6-diamidino-2-phenylindole staining), and calcofluor-staining moieties. More prolonged incubation in the spermidine-deficient medium leads to profound morphological changes including nuclear degeneration.
Figures



References
-
- Tabor C. W. & Tabor, H. (1984) Annu. Rev. Biochem. 53, 749-790. - PubMed
-
- Cohen S. S., (1998) A Guide to the Polyamines (Oxford Univ. Press, New York), pp. 1–595.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

