Substrate-dependent reversal of anion transport site orientation in the human red blood cell anion-exchange protein, AE1
- PMID: 12149479
- PMCID: PMC125063
- DOI: 10.1073/pnas.162402399
Substrate-dependent reversal of anion transport site orientation in the human red blood cell anion-exchange protein, AE1
Abstract
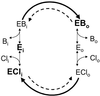
The tightly coupled, one-for-one exchange of anions mediated by the human red blood cell AE1 anion-exchange protein involves a ping-pong mechanism, in which AE1 alternates between a state with the anion-binding site facing inward toward the cytoplasm (Ei) and a state with the site facing outward toward the external medium (Eo). The conformational shift (Ei <--> Eo) is only permitted when a suitable substrate such as Cl(-) or HCO(3)(-) (B(-)) is bound. With no anions bound, or with Cl(-) bound, far more AE1 molecules are in the inward-facing than the outward-facing forms (Ei Eo, ECli EClo). We have constructed a model for CI(-)-B(-) exchange based on Cl(-)-Cl(-) and B(-)-B(-) exchange data, and have used it to predict the heteroexchange flux under extremely asymmetric conditions, with either all Cl(-) inside and all B(-) outside (Cli-Bo) or vice versa (Bi-Clo). The experimental values of the ratio of the exchange rate for Bi-Clo to that for Cli-Bo are only compatible with the model if the asymmetry of bicarbonate-loaded sites (A(B) = EBo/EBi) > 10, the opposite of the asymmetry for unloaded or Cl-loaded sites. Furthermore, the Eo form has a higher affinity for HCO(3)(-) than for Cl(-), whereas the Ei form has a higher affinity for Cl(-). The fact that this "passive" system exhibits changes in substrate selectivity with site orientation ("sidedness"), a characteristic usually associated with energy-coupled "active" pumps, suggests that changes in affinity with changes in sidedness are a more general property of transport proteins than previously thought.
Figures



Similar articles
-
Flufenamic acid senses conformation and asymmetry of human erythrocyte band 3 anion transport protein.Am J Physiol. 1989 Aug;257(2 Pt 1):C277-89. doi: 10.1152/ajpcell.1989.257.2.C277. Am J Physiol. 1989. PMID: 2764091
-
The noncompetitive inhibitor WW781 senses changes in erythrocyte anion exchanger (AE1) transport site conformation and substrate binding.J Gen Physiol. 2000 Feb;115(2):159-73. doi: 10.1085/jgp.115.2.159. J Gen Physiol. 2000. PMID: 10653894 Free PMC article.
-
Source of transport site asymmetry in the band 3 anion exchange protein determined by NMR measurements of external Cl- affinity.Biochemistry. 1996 Dec 3;35(48):15228-35. doi: 10.1021/bi961443b. Biochemistry. 1996. PMID: 8952471
-
Kinetics and mechanism of anion transport in red blood cells.Annu Rev Physiol. 1985;47:519-33. doi: 10.1146/annurev.ph.47.030185.002511. Annu Rev Physiol. 1985. PMID: 3922288 Review. No abstract available.
-
Molecular physiology of SLC4 anion exchangers.Exp Physiol. 2006 Jan;91(1):153-61. doi: 10.1113/expphysiol.2005.031765. Epub 2005 Oct 20. Exp Physiol. 2006. PMID: 16239253 Review.
Cited by
-
Inhibition of the Na/bicarbonate cotransporter NBCe1-A by diBAC oxonol dyes relative to niflumic acid and a stilbene.J Membr Biol. 2007 Feb;215(2-3):195-204. doi: 10.1007/s00232-007-9018-z. Epub 2007 Jun 20. J Membr Biol. 2007. PMID: 17578633
-
Hydrogen ion dynamics in human red blood cells.J Physiol. 2010 Dec 15;588(Pt 24):4995-5014. doi: 10.1113/jphysiol.2010.197392. Epub 2010 Oct 20. J Physiol. 2010. PMID: 20962000 Free PMC article.
-
Cell physiology and molecular mechanism of anion transport by erythrocyte band 3/AE1.Am J Physiol Cell Physiol. 2021 Dec 1;321(6):C1028-C1059. doi: 10.1152/ajpcell.00275.2021. Epub 2021 Oct 20. Am J Physiol Cell Physiol. 2021. PMID: 34669510 Free PMC article. Review.
-
A provisional transport mechanism for a chloride channel-type Cl-/H+ exchanger.Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):175-80. doi: 10.1098/rstb.2008.0138. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18977737 Free PMC article.
-
Separate ion pathways in a Cl-/H+ exchanger.J Gen Physiol. 2005 Dec;126(6):563-70. doi: 10.1085/jgp.200509417. J Gen Physiol. 2005. PMID: 16316975 Free PMC article.
References
-
- DeWeer P. (1992) in The Kidney: Physiology and Pathophysiology, eds. Seldin, D. W. & Giebisch, G. (Raven, New York), Vol. 2, pp. 93–112.
-
- Jennings M. L. (1992) in The Kidney: Physiology and Pathophysiology, eds. Seldin, D. W. & Giebisch, G. (Raven, New York), Vol. 2, pp. 113–145.
-
- Jennings M. L., Whitlock, J. & Shinde, A. (1998) Biochem. Cell Biol. 76, 807-813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

