A -1 ribosomal frameshift element that requires base pairing across four kilobases suggests a mechanism of regulating ribosome and replicase traffic on a viral RNA
- PMID: 12149516
- PMCID: PMC123222
- DOI: 10.1073/pnas.162223099
A -1 ribosomal frameshift element that requires base pairing across four kilobases suggests a mechanism of regulating ribosome and replicase traffic on a viral RNA
Abstract
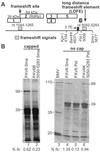
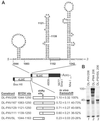
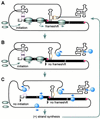
Programmed -1 ribosomal frameshifting is necessary for translation of the polymerase genes of many viruses. In addition to the consensus elements in the mRNA around the frameshift site, we found previously that frameshifting on Barley yellow dwarf virus RNA requires viral sequence located four kilobases downstream. By using dual luciferase reporter constructs, we now show that a predicted loop in the far downstream frameshift element must base pair to a bulge in a bulged stem loop adjacent to the frameshift site. Introduction of either two or six base mismatches in either the bulge or the far downstream loop abolished frameshifting, whereas mutations in both sites that restored base pairing reestablished frameshifting. Likewise, disruption of this base pairing abolished viral RNA replication in plant cells, and restoration of base pairing completely reestablished virus replication. We propose a model in which Barley yellow dwarf virus uses this and another long-distance base-pairing event required for cap-independent translation to allow the replicase copying from the 3' end to shut off translation of upstream ORFs and free the RNA of ribosomes to allow unimpeded replication. This would be a means of solving the "problem," common to positive strand RNA viruses, of competition between ribosomes and replicase for the same RNA template.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

