Multimodal quantal release at individual hippocampal synapses: evidence for no lateral inhibition
- PMID: 12151511
- PMCID: PMC6758134
- DOI: 10.1523/JNEUROSCI.22-15-06336.2002
Multimodal quantal release at individual hippocampal synapses: evidence for no lateral inhibition
Abstract
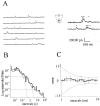
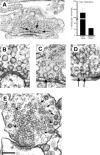
Most CNS synapses investigated thus far contain a large number of vesicles docked at the active zone, possibly forming individual release sites. At the present time, it is unclear whether these vesicles can be discharged independently of one another. To investigate this problem, we recorded miniature excitatory currents by whole-cell and single-synapse recordings from CA3-CA1 hippocampal neurons and analyzed their stochastic properties. In addition, spontaneous release was investigated by ultrastructural analysis of quickly frozen synapses, revealing vesicle intermediates in docking and spontaneous fusion states. In these experiments, no signs of inhibitory interactions between quanta could be detected up to 1 msec from the previous discharge. This suggests that exocytosis at one site does not per se inhibit vesicular fusion at neighboring sites. At longer intervals, the output of quanta diverged from a random memoryless Poisson process because of the presence of a bursting component. The latter, which could not be accounted for by random coincidences, was independent of Ca2+ elevations in the cytosol, whether from Ca2+ flux through the plasma membrane or release from internal stores. Results of these experiments, together with the observation of spontaneous pairs of omega profiles at the active zone, suggest that multimodal release is produced by an enduring activation of an integrated cluster of release sites.
Figures





References
-
- Antonova I, Arancio O, Trillat AC, Wang HG, Zablow L, Udo H, Kandel ER, Hawkins RD. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science. 2001;294:1547–1550. - PubMed
-
- Auger C, Marty A. A heterogeneity of functional synaptic parameters among single release sites. Neuron. 1997;19:139–150. - PubMed
-
- Augustine GJ, Charlton MP, Smith SJ. Ca2+ action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
