Postsynaptic targeting of alternative postsynaptic density-95 isoforms by distinct mechanisms
- PMID: 12151521
- PMCID: PMC6758133
- DOI: 10.1523/JNEUROSCI.22-15-06415.2002
Postsynaptic targeting of alternative postsynaptic density-95 isoforms by distinct mechanisms
Abstract
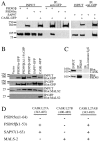
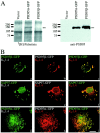
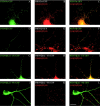
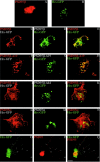
Members of the postsynaptic density-95 (PSD95)/synapse-associated protein-90 (SAP90) family of scaffolding proteins contain a common set of modular protein interaction motifs including PDZ (postsynaptic density-95/Discs large/zona occludens-1), Src homology 3, and guanylate kinase domains, which regulate signaling and plasticity at excitatory synapses. We report that N-terminal alternative splicing of PSD95 generates an isoform, PSD95beta that contains an additional "L27" motif, which is also present in SAP97. Using yeast two hybrid and coimmunoprecipitation assays, we demonstrate that this N-terminal L27 domain of PSD-95beta, binds to an L27 domain in the membrane-associated guanylate kinase calcium/calmodulin-dependent serine kinase, and to Hrs, an endosomal ATPase that regulates vesicular trafficking. By transfecting heterologous cells and hippocampal neurons, we find that interactions with the L27 domain regulate synaptic clustering of PSD95beta. Disrupting Hrs-regulated early endosomal sorting in hippocampal neurons selectively blocks synaptic clustering of PSD95beta but does not interfere with trafficking of the palmitoylated isoform, PSD95alpha. These studies identify molecular and functional heterogeneity in synaptic PSD95 complexes and reveal critical roles for L27 domain interactions and Hrs regulated vesicular trafficking in postsynaptic protein clustering.
Figures




References
-
- Bean AJ, Seifert R, Chen YA, Sacks R, Scheller RH. Hrs-2 is an ATPase implicated in calcium-regulated secretion. Nature. 1997;385:826–829. - PubMed
-
- Bean AJ, Davanger S, Chou MF, Gerhardt B, Tsujimoto S, Chang Y. Hrs-2 regulates receptor-mediated endocytosis via interactions with Eps15. J Biol Chem. 2000;275:15271–15278. - PubMed
-
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α-1 syntrophin mediated by PDZ motifs. Cell. 1996a;84:757–767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
