Distribution of a lysosomal enzyme in the adult brain by axonal transport and by cells of the rostral migratory stream
- PMID: 12151523
- PMCID: PMC6758166
- DOI: 10.1523/JNEUROSCI.22-15-06437.2002
Distribution of a lysosomal enzyme in the adult brain by axonal transport and by cells of the rostral migratory stream
Abstract
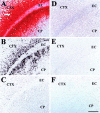
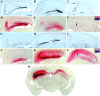
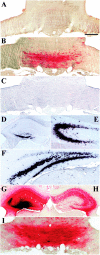
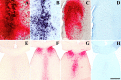
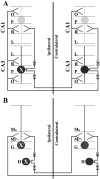
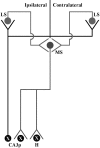
A portion of the lysosomal enzymes produced by cells is secreted, diffuses through extracellular spaces, and can be taken up by distal cells via mannose-6-phosphate receptor-mediated endocytosis. This provides the basis for treating lysosomal storage diseases, many of which affect the CNS. Normal enzyme secreted from a cluster of genetically corrected cells has been shown to reverse storage lesions in a zone of surrounding brain tissue in mouse disease models. However, low levels of enzyme activity and reduction of storage lesions also have been observed at sites in the brain that may not be explained by a contiguous gradient of secreted enzyme diffusing away from the genetically corrected cells. No direct evidence for alternative mechanisms of enzyme transport has been shown, and little is understood about the intracellular movement of lysosomal enzymes in neurons. We investigated whether axonal transport could occur, by expressing an eukaryotic lysosomal enzyme that can be visualized in tissue sections (beta-glucuronidase) in brain structures that have defined axonal connections to other structures. This resulted in the transfer of enzyme to, and a reversal of storage lesions in, neurons that project to the gene expression site, but not in nearby structures that would have been corrected if the effect had been mediated by diffusion. In addition, transduction of cells in the subventricular zone resulted in the uptake of beta-glucuronidase by cells entering the rostral migratory stream. Gene transfer to specific neuronal circuits or cells in migratory pathways may facilitate delivery to the global brain lesions found in these disorders.
Figures



Similar articles
-
Widespread correction of lysosomal storage in the mucopolysaccharidosis type VII mouse brain with a herpes simplex virus type 1 vector expressing beta-glucuronidase.Mol Ther. 2006 May;13(5):859-69. doi: 10.1016/j.ymthe.2005.12.017. Epub 2006 Mar 3. Mol Ther. 2006. PMID: 16515890
-
Long-term and significant correction of brain lesions in adult mucopolysaccharidosis type VII mice using recombinant AAV vectors.Mol Ther. 2000 Jan;1(1):63-70. doi: 10.1006/mthe.1999.0005. Mol Ther. 2000. PMID: 10933913
-
Decreased lysosomal storage in the adult MPS VII mouse brain in the vicinity of grafts of retroviral vector-corrected fibroblasts secreting high levels of beta-glucuronidase.Nat Med. 1997 Jul;3(7):771-4. doi: 10.1038/nm0797-771. Nat Med. 1997. PMID: 9212105
-
Murine mucopolysaccharidosis type VII: the impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease.J Inherit Metab Dis. 1998 Aug;21(5):575-86. doi: 10.1023/a:1005423222927. J Inherit Metab Dis. 1998. PMID: 9728337 Review.
-
New strategies for enzyme replacement therapy for lysosomal storage diseases.Rejuvenation Res. 2010 Apr-Jun;13(2-3):229-36. doi: 10.1089/rej.2009.0920. Rejuvenation Res. 2010. PMID: 20345279 Free PMC article. Review.
Cited by
-
Ex vivo gene therapy using patient iPSC-derived NSCs reverses pathology in the brain of a homologous mouse model.Stem Cell Reports. 2015 May 12;4(5):835-46. doi: 10.1016/j.stemcr.2015.02.022. Epub 2015 Apr 9. Stem Cell Reports. 2015. PMID: 25866157 Free PMC article.
-
Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment.Mol Ther. 2014 Mar;22(3):554-566. doi: 10.1038/mt.2013.237. Epub 2013 Oct 8. Mol Ther. 2014. PMID: 24100640 Free PMC article.
-
AAV-mediated gene delivery in a feline model of Sandhoff disease corrects lysosomal storage in the central nervous system.ASN Neuro. 2015 Apr 13;7(2):1759091415569908. doi: 10.1177/1759091415569908. Print 2015 Mar-Apr. ASN Neuro. 2015. PMID: 25873306 Free PMC article.
-
Merits of combination cortical, subcortical, and cerebellar injections for the treatment of Niemann-Pick disease type A.Mol Ther. 2012 Oct;20(10):1893-901. doi: 10.1038/mt.2012.118. Epub 2012 Jul 24. Mol Ther. 2012. PMID: 22828503 Free PMC article.
-
AAV gene therapy for Tay-Sachs disease.Nat Med. 2022 Feb;28(2):251-259. doi: 10.1038/s41591-021-01664-4. Epub 2022 Feb 10. Nat Med. 2022. PMID: 35145305 Free PMC article.
References
-
- Achord D, Brot F, Gonzalez-Noriega A, Sly W, Stahl P. Human β-glucuronidase. II. Fate of infused human placental β-glucuronidase in the rat. Pediatr Res. 1977;11:816–822. - PubMed
-
- Alexander IE, Russell DW, Spence AM, Miller AD. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by adeno-associated virus vectors. Hum Gene Ther. 1996;7:841–850. - PubMed
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. - PubMed
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
-
- Barthel LK, Raymond PA. In situ hybridization studies of retinal neurons. Methods Enzymol. 2000;316:579–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
