Localization of the activation gate for small conductance Ca2+-activated K+ channels
- PMID: 12151529
- PMCID: PMC6758173
- DOI: 10.1523/JNEUROSCI.22-15-06499.2002
Localization of the activation gate for small conductance Ca2+-activated K+ channels
Abstract
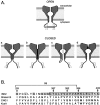
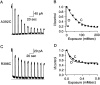
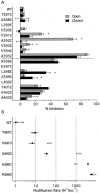
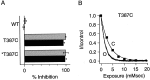
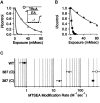
Small conductance Ca2+-activated K+ (SK) channels open in response to increased cytosolic Ca2+ and contribute to the afterhyperpolarization in many excitable cell types. Opening of SK channels is initiated by Ca2+ binding to calmodulin that is bound to the C terminus of the channel. Based on structural information, a chemomechanical gating model has been proposed in which the chemical energy derived from Ca2+ binding is transduced into a mechanical force that restructures the protein to allow K+ ion conduction through the pore. However, the residues that comprise the physical gate of the SK channels have not been identified. In voltage-gated K+ (Kv) channels, access to the inner vestibule is controlled by a bundle crossing formed by the intracellular end of the sixth transmembrane domain (S6) of each of the four channel subunits. Probing SK channels with internally applied quaternary amines suggests that the inner vestibules of Kv and SK channels share structural similarity. Using substituted cysteine accessibility mutagenesis, the relatively large molecule [2-(trimethylammonium)] methanethiosulfonate accessed positions near the putative bundle crossing more rapidly in the open than the closed state but did not modify S6 positions closer to the selectivity filter. In contrast, the smaller compound, 2-(aminoethyl) methanethiosulfonate (MTSEA), modified a position predicted to lie in the lumen immediately intracellular to the selectivity filter equivalently in the open and closed states. The pore blocker tetrabutylammonium impeded MTSEA access to this position in both open and closed channels. The results suggest that the SK channel gate is not formed by the cytoplasmic end of S6 but resides deep in the channel pore in or near the selectivity filter.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
