Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model
- PMID: 12151531
- PMCID: PMC6758154
- DOI: 10.1523/JNEUROSCI.22-15-06515.2002
Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model
Abstract
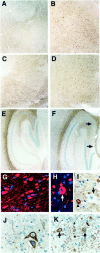
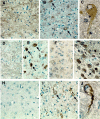
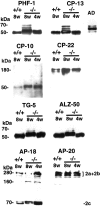
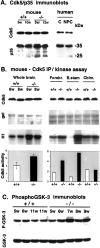
NPC-1 gene mutations cause Niemann-Pick type C (NPC), a neurodegenerative storage disease resulting in premature death in humans. Spontaneous mutation of the NPC-1 gene in mice generates a similar phenotype, usually with death ensuing by 12 weeks of age. Both human and murine NPC are characterized neuropathologically by ballooned neurons distended with lipid storage, axonal spheroid formation, demyelination, and widespread neuronal loss. To elucidate the biochemical mechanism underlying this neuropathology, we have investigated the phosphorylation of neuronal cytoskeletal proteins in the brains of npc-1 mice. A spectrum of antibodies against phosphorylated epitopes in neurofilaments (NFs) and MAP2 and tau were used in immunohistochemical and immunoblotting analyses of 4- to 12-week-old mice. Multiple sites in NFs, MAP2, and tau were hyperphosphorylated as early as 4 weeks of age and correlated with a significant increase in activity of the cyclin-dependent kinase 5 (cdk5) and accumulation of its more potent activator, p25, a proteolytic fragment of p35. At 5 weeks of age, the development of axonal spheroids was noted in the pons. p25 and cdk5 coaccumulated with hyperphosphorylated cytoskeletal proteins in axon spheroids. These various abnormalities escalated with each additional week of age, spreading to other regions of the brainstem, basal ganglia, cerebellum, and eventually, the cortex. Our data suggest that focal deregulation of cdk5/p25 in axons leads to cytoskeletal abnormalities and eventual neurodegeneration in NPC. The npc-1 mouse is a valuable in vivo model for determining how and when cdk5 becomes deregulated and whether cdk5 inhibitors would be useful in blocking NPC neurodegeneration.
Figures


Similar articles
-
Cyclin-dependent kinase inhibitors attenuate protein hyperphosphorylation, cytoskeletal lesion formation, and motor defects in Niemann-Pick Type C mice.Am J Pathol. 2004 Sep;165(3):843-53. doi: 10.1016/S0002-9440(10)63347-0. Am J Pathol. 2004. PMID: 15331409 Free PMC article.
-
p35/p25 is not essential for tau and cytoskeletal pathology or neuronal loss in Niemann-Pick type C disease.J Neurosci. 2006 Mar 8;26(10):2738-44. doi: 10.1523/JNEUROSCI.4834-05.2006. J Neurosci. 2006. PMID: 16525053 Free PMC article.
-
Aberrant activation of Cdc2/cyclin B1 is involved in initiation of cytoskeletal pathology in murine Niemann-Pick disease type C.J Huazhong Univ Sci Technolog Med Sci. 2017 Oct;37(5):732-739. doi: 10.1007/s11596-017-1796-7. Epub 2017 Oct 20. J Huazhong Univ Sci Technolog Med Sci. 2017. PMID: 29058287
-
The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer's pathology.Eur J Biochem. 2001 Mar;268(6):1518-27. doi: 10.1046/j.1432-1033.2001.02024.x. Eur J Biochem. 2001. PMID: 11248668 Review.
-
Cyclin-dependent protein kinase 5 (Cdk5) and the regulation of neurofilament metabolism.Eur J Biochem. 2001 Mar;268(6):1534-46. Eur J Biochem. 2001. PMID: 11248670 Review.
Cited by
-
Mechanism of CDK5 activation revealed by steered molecular dynamics simulations and energy calculations.J Mol Model. 2010 Jun;16(6):1159-68. doi: 10.1007/s00894-009-0629-4. Epub 2009 Dec 15. J Mol Model. 2010. PMID: 20013135
-
Cyclin-dependent kinase inhibitors attenuate protein hyperphosphorylation, cytoskeletal lesion formation, and motor defects in Niemann-Pick Type C mice.Am J Pathol. 2004 Sep;165(3):843-53. doi: 10.1016/S0002-9440(10)63347-0. Am J Pathol. 2004. PMID: 15331409 Free PMC article.
-
Packaging and functional identification of recombinant adeno-associated virus encoding cdc2-siRNA.J Huazhong Univ Sci Technolog Med Sci. 2008 Dec;28(6):626-9. doi: 10.1007/s11596-008-0602-y. Epub 2008 Dec 24. J Huazhong Univ Sci Technolog Med Sci. 2008. PMID: 19107353
-
An unusual member of the Cdk family: Cdk5.Cell Mol Neurobiol. 2008 May;28(3):351-69. doi: 10.1007/s10571-007-9242-1. Epub 2008 Jan 8. Cell Mol Neurobiol. 2008. PMID: 18183483 Free PMC article. Review.
-
Zebrafish Rohon-Beard neuron development: cdk5 in the midst.Neurochem Res. 2009 Jun;34(6):1129-37. doi: 10.1007/s11064-008-9885-4. Epub 2008 Dec 9. Neurochem Res. 2009. PMID: 19067160 Free PMC article. Review.
References
-
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA. 2000;97:2910–2915. - PMC - PubMed
-
- Auer IA, Schmidt ML, Lee VM, Curry B, Suzuki K, Shin RW, Pentchev PG, Carstea ED, Trojanowski JQ. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer's disease. Acta Neuropathol. 1995;90:547–551. - PubMed
-
- Berling B, Wille H, Roll B, Mandelkow EM, Garner C, Mandelkow E. Phosphorylation of microtubule-associated proteins MAP2a,b and MAP2c at Ser136 by proline-directed kinases in vivo and in vitro. Eur J Cell Biol. 1994;64:120–130. - PubMed
-
- Bialkowska K, Kulkarni S, Du X, Goll DE, Saido TC, Fox JE. Evidence that beta3 integrin-induced Rac activation involves the calpain-dependent formation of integrin clusters that are distinct from the focal complexes and focal adhesions that form as Rac and RhoA become active. J Cell Biol. 2000;151:685–696. - PMC - PubMed
-
- Binder LI, Frankfurter A, Rebhun LI. Differential localization of MAP-2 and tau in mammalian neurons in situ. Ann NY Acad Sci. 1986;466:145–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
