Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron
- PMID: 12151542
- PMCID: PMC6758126
- DOI: 10.1523/JNEUROSCI.22-15-06631.2002
Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron
Abstract
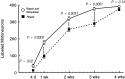
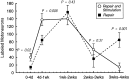
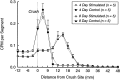
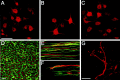
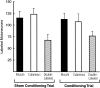
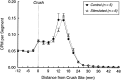
Motoneurons reinnervate the distal stump at variable rates after peripheral nerve transection and suture. In the rat femoral nerve model, reinnervation is already substantial 3 weeks after repair, but is not completed for an additional 7 weeks. However, this "staggered regeneration" can be temporally compressed by application of 20 Hz electrical stimulation to the nerve for 1 hr. The present experiments explore two possible mechanisms for this stimulation effect: (1) synchronization of distal stump reinnervation and (2) enhancement of regeneration speed. The first possibility was investigated by labeling all motoneurons that have crossed the repair at intervals from 4 d to 4 weeks after rat femoral nerve transection and suture. Although many axons did not cross until 3-4 weeks after routine repair, stimulation significantly increased the number crossing at 4 and 7 d, with only a few crossing after 2 weeks. Regeneration speed was studied by radioisotope labeling of transported proteins and by anterograde labeling of regenerating axons, and was not altered by stimulation. Attempts to condition the neuron by stimulating the femoral nerve 1 week before injury were also without effect. Electrical stimulation thus promotes the onset of motor axon regeneration without increasing its speed. This finding suggests a combined approach to improving the outcome of nerve repair, beginning with stimulation to recruit all motoneurons across the repair, followed by other treatments to speed and prolong axonal elongation.
Figures

References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB rnRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000a;12:4381–4390. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
