Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity
- PMID: 12151550
- PMCID: PMC6758156
- DOI: 10.1523/JNEUROSCI.22-15-06713.2002
Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity
Abstract
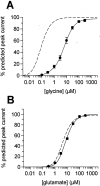
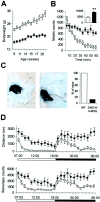
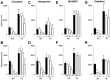
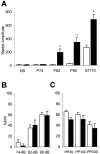
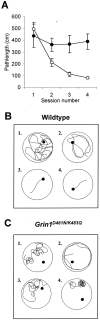
NMDA receptor hypofunction has been implicated in the pathophysiology of schizophrenia, and pharmacological and genetic approaches have been used to model such dysfunction. We previously have described two mouse lines carrying point mutations in the NMDA receptor glycine binding site, Grin1(D481N) and Grin1(K483Q), which exhibit 5- and 86-fold reductions in receptor glycine affinity, respectively. Grin1(D481N) animals exhibit a relatively mild phenotype compatible with a moderate reduction in NMDA receptor function, whereas Grin1(K483Q) animals die shortly after birth. In this study we have characterized compound heterozygote Grin1(D481N/K483Q) mice, which are viable and exhibited biphasic NMDA receptor glycine affinities compatible with the presence of each of the two mutated alleles. Grin1(D481N/K483Q) mice exhibited a marked NMDA receptor hypofunction revealed by deficits in hippocampal long-term potentiation, which were rescued by the glycine site agonist d-serine, which also facilitated NMDA synaptic currents in mutant, but not in wild-type, mice. Analysis of striatal monoamine levels revealed an apparent dopaminergic and serotonergic hyperfunction. Behaviorally, Grin1(D481N/K483Q) mice were insensitive to acute dizocilpine pretreatment and exhibited increased startle response but normal prepulse inhibition. Most strikingly, mutant mice exhibited a sustained, nonhabituating hyperactivity and increased stereotyped behavior that were resistant to suppression by antipsychotics and the benzodiazepine site agonist Zolpidem. They also displayed a disruption of nest building behavior and were unable to perform a cued learning paradigm in the Morris water maze. We speculate that the severity of NMDA receptor hypofunction in these mice may account for their profound behavioral phenotype and insensitivity to antipsychotics.
Figures


References
-
- Bristow LJ, Hutson PH, Kulagowski JJ, Leeson PD, Matheson S, Murray F, Rathbone D, Saywell KL, Thorn L, Watt AP, Tricklebank MD. Anticonvulsant and behavioural profile of L-701,324, a potent, orally active antagonist at the glycine modulatory site on the N-methyl-d-aspartate receptor complex. J Pharmacol Exp Ther. 1996;279:492–501. - PubMed
-
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. - PubMed
-
- Carlsson ML, Martin P, Nilsson M, Sorensen SM, Carlsson A, Waters S, Waters N. The 5-HT2A receptor antagonist M100907 is more effective in countering NMDA antagonist- than dopamine agonist-induced hyperactivity in mice. J Neural Transm. 1999;106:123–129. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
