Human small intestinal mucosa harbours a small population of cytolytically active CD8+ alphabeta T lymphocytes
- PMID: 12153510
- PMCID: PMC1782753
- DOI: 10.1046/j.1365-2567.2002.01461.x
Human small intestinal mucosa harbours a small population of cytolytically active CD8+ alphabeta T lymphocytes
Abstract
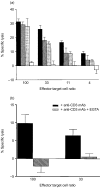
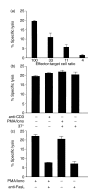
Intraepithelial lymphocytes (IEL) in normal human small intestine exhibit cytotoxicity. This study was undertaken to characterize the effector cells and their mode of action. Freshly isolated jejunal IEL and lamina propria lymphocytes (LPL), as well as IEL and LPL depleted of CD4+, CD8+ and T-cell receptor (TCR)-gammadelta+ cells were used as effector cells in anti-CD3-mediated redirected cytotoxicity against a murine FcgammaR-expressing cell line. Effector cell frequencies were estimated by effector to target cell titration and limiting dilution. The capacity of IEL and LPL to kill a Fas-expressing human T-cell line was also analysed. T-cell subsets were analysed for perforin, granzyme B, Fas-ligand (FasL), tumour necrosis factor-alpha (TNF-alpha) and TNF-related apoptosis inducing ligand (TRAIL) mRNA expression by reverse transcription-polymerase chain reaction (RT-PCR). Frequencies of IEL expressing the perforin and FasL proteins were determined by immunomorphometry. Both IEL and LPL exhibited significant Ca2+-dependent, anti-CD3-mediated cytotoxicity, approximately 30% specific lysis at the effector to target cell ratio 100. The cytotoxic cells constituted, however, only a small fraction of IEL and LPL ( approximately 0.01%). CD8+ TCR-alphabeta+ cells accounted for virtually all the cytotoxicity and expressed mRNA for all five cytotoxic proteins. The frequency of granzyme B-expressing samples was higher in CD8+ cells than in CD4+ cells (P<0.05 and <0.01 for IEL and LPL, respectively). In addition, both IEL and LPL exhibited significant spontaneous anti-CD3-independent cytotoxicity against Fas-expressing human T cells. This killing was mediated by Fas-FasL interaction. On average, 2-3% of the IEL expressed perforin and FasL. We speculate that CD8+ memory cells accumulate in the jejunal mucosa and that the CD8+ TCR-alphabeta+ lymphocytes executing TCR/CD3-mediated, Ca2+-dependent cytotoxicity are classical cytotoxic T lymphocytes 'caught in the act' of eliminating infected epithelial cells through perforin/granzyme exocytosis. The observed Fas/FasL-mediated cytotoxicity may be a reflection of ongoing down-regulation of local immune responses by 'activation-induced cell death'.
Figures



Similar articles
-
Interleukin-12 up-regulates perforin- and Fas-mediated lymphokine-activated killer activity by intestinal intraepithelial lymphocytes.Clin Exp Immunol. 2004 Nov;138(2):259-65. doi: 10.1111/j.1365-2249.2004.02614.x. Clin Exp Immunol. 2004. PMID: 15498035 Free PMC article.
-
Cytolytic capabilities of lamina propria and intraepithelial lymphocytes in normal and chronically inflamed human intestine.Scand J Immunol. 2004 Jul-Aug;60(1-2):167-77. doi: 10.1111/j.0300-9475.2004.01434.x. Scand J Immunol. 2004. PMID: 15238086
-
Unique properties of a cytotoxic CD4+CD8+ intraepithelial T-cell line established from the mouse intestinal epithelium.Microbiol Immunol. 1994;38(3):191-9. doi: 10.1111/j.1348-0421.1994.tb01764.x. Microbiol Immunol. 1994. PMID: 8078424
-
The interaction of intestinal epithelial cells and intraepithelial lymphocytes in host defense.Immunol Res. 1999;20(3):219-35. doi: 10.1007/BF02790405. Immunol Res. 1999. PMID: 10741862 Review.
-
Escaping Death: How Cancer Cells and Infected Cells Resist Cell-Mediated Cytotoxicity.Front Immunol. 2022 Mar 23;13:867098. doi: 10.3389/fimmu.2022.867098. eCollection 2022. Front Immunol. 2022. PMID: 35401556 Free PMC article. Review.
Cited by
-
Noncontaminated dietary oats may hamper normalization of the intestinal immune status in childhood celiac disease.Clin Transl Gastroenterol. 2014 Jun 26;5(6):e58. doi: 10.1038/ctg.2014.9. Clin Transl Gastroenterol. 2014. PMID: 24964993 Free PMC article.
-
Human intraepithelial lymphocytes.Mucosal Immunol. 2018 Sep;11(5):1281-1289. doi: 10.1038/s41385-018-0016-5. Epub 2018 Apr 20. Mucosal Immunol. 2018. PMID: 29674648 Free PMC article. Review.
-
Interleukin-12 up-regulates perforin- and Fas-mediated lymphokine-activated killer activity by intestinal intraepithelial lymphocytes.Clin Exp Immunol. 2004 Nov;138(2):259-65. doi: 10.1111/j.1365-2249.2004.02614.x. Clin Exp Immunol. 2004. PMID: 15498035 Free PMC article.
-
Characterization of Bovine Intraepithelial T Lymphocytes in the Gut.Pathogens. 2023 Sep 19;12(9):1173. doi: 10.3390/pathogens12091173. Pathogens. 2023. PMID: 37764981 Free PMC article.
-
Aberrant extrathymic T cell receptor gene rearrangement in the small intestinal mucosa: a risk factor for coeliac disease?Gut. 2009 Feb;58(2):189-95. doi: 10.1136/gut.2007.125526. Epub 2008 Feb 25. Gut. 2009. PMID: 18299319 Free PMC article.
References
-
- Lundqvist C, Baranov V, Hammarström S, Athlin L, Hammarström ML. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995;7:1473–87. - PubMed
-
- Camerini V, Panwala C, Kronenberg M. Regional specialization of the mucosal immune system: Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol. 1993;151:1765–75. - PubMed
-
- Guy-Grand D, Malassis-Seris M, Briottet C, Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991;173:1549–52. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

