Endotoxin, but not platelet-activating factor, activates nuclear factor-kappaB and increases IkappaBalpha and IkappaBbeta turnover in enterocytes
- PMID: 12153521
- PMCID: PMC1782741
- DOI: 10.1046/j.1365-2567.2002.01453.x
Endotoxin, but not platelet-activating factor, activates nuclear factor-kappaB and increases IkappaBalpha and IkappaBbeta turnover in enterocytes
Abstract
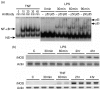
Bacterial endotoxin (lipopolysaccharide; LPS) and platelet-activating factor (PAF) are important triggers of bowel inflammation and injury. We have previously shown that LPS activates the transcription factor nuclear factor (NF)-kappaB in the intestine, which up-regulates many pro-inflammatory genes. This effect partly depends on neutrophils and endogenous PAF. However, whether LPS and PAF directly activate NF-kappaB in enterocytes remains controversial. In this study, we first investigated the effect of LPS and PAF on NF-kappaB activation in IEC-6 (a non-transformed rat small intestinal crypt cell line) cells, by electrophoresis mobility shift assay and supershift, and found that LPS, but not PAF, activates NF-kappaB mostly as p50-p65 heterodimers. The effect was slower than tumour necrosis factor (TNF). Both LPS and TNF induce the expression of the NF-kappaB-dependent gene inducible nitric oxide synthase (iNOS), which occurs subsequent to NF-kappaB activation. We then examined the effect of LPS and TNF on the inhibitory molecules IkappaBalpha and IkappaBbeta. We found that TNF causes rapid degradation of IkappaBalpha and IkappaBbeta. In contrast, LPS did not change the levels of IkappaBalpha and IkappaBbeta up to 4 hr (by Western blot). However, in the presence of cycloheximide, there was a slow reduction of IkappaBalpha and IkappaBbeta, which disappeared almost completely at 4 hr. These observations suggest that LPS causes slow degradation and synthesis of IkappaBalpha and IkappaBbeta and therefore activates NF-kappaBeta via at least two mechanisms: initially, through an IkappaB-independent mechanism, and later, via an increased turnover of the inhibitor IkappaB. NF-kappaBeta activation precedes the gene expression of iNOS (assayed by reverse transcription-polymerase chain reaction), suggesting that LPS up-regulates iNOS via this transcription factor.
Figures
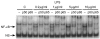
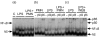
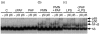


Similar articles
-
Lipopolysaccharide induces CXCL2/macrophage inflammatory protein-2 gene expression in enterocytes via NF-kappaB activation: independence from endogenous TNF-alpha and platelet-activating factor.Immunology. 2006 Jun;118(2):153-63. doi: 10.1111/j.1365-2567.2006.02344.x. Immunology. 2006. PMID: 16771850 Free PMC article.
-
Neuronal nitric oxide synthase (NOS) regulates the expression of inducible NOS in rat small intestine via modulation of nuclear factor kappa B.FASEB J. 2001 Feb;15(2):439-46. doi: 10.1096/fj.99-0343com. FASEB J. 2001. PMID: 11156959
-
Suppression of lipopolysaccharide-induced nitric oxide synthase expression by platelet-activating factor receptor antagonists in the rat liver and cultured rat Kupffer cells.Hepatology. 1999 Nov;30(5):1206-14. doi: 10.1002/hep.510300530. Hepatology. 1999. PMID: 10534342
-
Novel insight into molecular mechanism of endotoxin shock: biochemical analysis of LPS receptor signaling in a cell-free system targeting NF-kappaB and regulation of cytokine production/action through beta2 integrin in vivo.J Leukoc Biol. 1996 Feb;59(2):145-51. doi: 10.1002/jlb.59.2.145. J Leukoc Biol. 1996. PMID: 8603986 Review.
-
Stimulation of NF-kappa B activation and gene expression by platelet-activating factor.Adv Exp Med Biol. 1996;416:143-51. doi: 10.1007/978-1-4899-0179-8_24. Adv Exp Med Biol. 1996. PMID: 9131140 Review.
Cited by
-
Protective effects of carbon monoxide-releasing molecule-2 on the barrier function of intestinal epithelial cells.PLoS One. 2014 Aug 7;9(8):e104032. doi: 10.1371/journal.pone.0104032. eCollection 2014. PLoS One. 2014. PMID: 25101775 Free PMC article.
-
Despite minimal hemodynamic alterations endotoxemia modulates NOS and p38-MAPK phosphorylation via metalloendopeptidases.Mol Cell Biochem. 2004 Oct;265(1-2):47-56. doi: 10.1023/b:mcbi.0000044314.29395.fb. Mol Cell Biochem. 2004. PMID: 15543933 Clinical Trial.
-
No longer an innocent bystander: epithelial toll-like receptor signaling in the development of mucosal inflammation.Mol Med. 2008 Sep-Oct;14(9-10):645-59. doi: 10.2119/2008-00035.Gribar. Mol Med. 2008. PMID: 18584047 Free PMC article. Review.
-
Platelet-activating factor induces the processing of nuclear factor-kappaB p105 into p50, which mediates acute bowel injury in mice.Am J Physiol Gastrointest Liver Physiol. 2009 Jul;297(1):G76-81. doi: 10.1152/ajpgi.00053.2009. Epub 2009 May 21. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19460845 Free PMC article.
-
Lipopolysaccharide induces CXCL2/macrophage inflammatory protein-2 gene expression in enterocytes via NF-kappaB activation: independence from endogenous TNF-alpha and platelet-activating factor.Immunology. 2006 Jun;118(2):153-63. doi: 10.1111/j.1365-2567.2006.02344.x. Immunology. 2006. PMID: 16771850 Free PMC article.
References
-
- Hsueh W, Gonzalez-Crussi F, Arroyave JL. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987;1:403–5. - PubMed
-
- Sun XM, Qu XW, Huang W, Granger DN, Bree M, Hsueh W. Role of leukocyte beta 2-integrin in PAF-induced shock and intestinal injury. Am J Physiol. 1996;270:G184–G190. - PubMed
-
- Couturier C, Jahns G, Kazatchkine MD, Haeffner-Cavaillon N. Membrane molecules which trigger the production of interleukin-1 and tumor necrosis factor-alpha by lipopolysaccharide-stimulated human monocytes. Eur J Immunol. 1992;22:1461–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

