Effect on endometrium of long term treatment with continuous combined oestrogen-progestogen replacement therapy: follow up study
- PMID: 12153918
- PMCID: PMC117635
- DOI: 10.1136/bmj.325.7358.239
Effect on endometrium of long term treatment with continuous combined oestrogen-progestogen replacement therapy: follow up study
Abstract
Objective: To determine effects of five years of treatment with an oral continuous combined regimen of 2 mg 17beta-oestradiol and 1 mg norethisterone acetate on endometrial histology in postmenopausal women.
Design: Follow up study in postmenopausal women.
Setting: 31 menopause clinics in the United Kingdom.
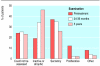
Participants: 534 postmenopausal women, all with an intact uterus, who had completed nine months of treatment with oral continuous combined 2 mg 17beta-oestradiol and 1 mg norethisterone acetate agreed to take part in a long term follow up study. Women were assigned to different groups on the basis of the treatment status immediately before entering the original study: 360 women had taken sequential oestrogen-progestogen hormone replacement therapy, 164 had taken no hormone replacement therapy, and 10 had taken unopposed oestrogen therapy.
Methods: Endometrial aspiration specimens were taken before the women started the continuous combined regimen, after 9 and 24-36 months, and at the end of the five year treatment period or on withdrawal from the study.
Main outcome measure: Results of endometrial histology.
Results: The duration of treatment with continuous combined hormone replacement therapy was 4.4 (range 1.1-5.9) years. Data on endometrial specimens were available for 526 women after nine months of treatment, 465 women after 24-36 months of treatment, and 398 women who completed the five years treatment (345 women) or were withdrawn between the two latter visits for biopsies (53 women). No cases of endometrial hyperplasia or malignancy were detected at biopsy; 69% of women had an endometrium classified as atrophic or unassessable on completion of the study or withdrawal from it. Before the continuous combined therapy was started, complex hyperplasia was detected in 21 women who had taken sequential hormone replacement therapy before the study and in one who had taken unopposed oestrogen. All of these women had normal results on histological examination of endometrial tissue after nine months of treatment with continuous combined hormone replacement therapy, and hyperplasia did not recur after up to five years of treatment.
Conclusions: Long term treatment (for up to five years) with continuous combined hormone replacement therapy containing oestradiol 2 mg and norethisterone 1 mg daily was associated with neither endometrial hyperplasia nor malignancy. In women who had complex hyperplasia during previous sequential or unopposed regimens, the endometrium returned to normal during treatment with continuous combined hormone replacement therapy. These findings provide reassurance about the long term safety of this continuous combined regimen in terms of the endometrium.
Figures
Comment in
-
Continuous combined hormone replacement therapy and endometrial hyperplasia.BMJ. 2002 Aug 3;325(7358):231-2. doi: 10.1136/bmj.325.7358.231. BMJ. 2002. PMID: 12153905 Free PMC article. No abstract available.
References
-
- Sturdee DW, Barlow DH, Ulrich LG, Wells M, Gydesen H, Campbell M, et al. Is the timing of withdrawal bleeding a guide to endometrial safety during sequential oestrogen-progestagen replacement therapy? Lancet. 1994;344:979–982. - PubMed
-
- Sturdee DW, Ulrich LG, Barlow DH, Wells M, Campbell MJ, Vessey MP, et al. The endometrial response to sequential and continuous combined oestrogen-progestogen replacement therapy. Br J Obstet Gynaecol. 2000;107:1392–1400. - PubMed
-
- Beresford SAA, Weiss NS, Voigt LF, McKnight B. Risk of endometrial cancer in relation to use of oestrogen combined with cyclic progestagen therapy in postmenopausal women. Lancet. 1997;349:458–461. - PubMed
-
- Weiderpass E, Adami HO, Baron JA, Magnusson C, Bergstrom R, Lindgren A, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst. 1999;91:1131–1137. - PubMed
-
- Ravnikar VA. Barriers for taking long-term hormone replacement therapy: why do women not adhere to therapy? Eur Menopause J. 1996;3:90–93.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical