Short-term cardiovascular oscillations in man: measuring and modelling the physiologies
- PMID: 12154170
- PMCID: PMC2290446
- DOI: 10.1113/jphysiol.2002.017483
Short-term cardiovascular oscillations in man: measuring and modelling the physiologies
Abstract
Research into cardiovascular variabilities intersects both human physiology and quantitative modelling. This is because respiratory and Mayer wave (or 10 s) cardiovascular oscillations represent the integrated control of a system through both autonomic branches by systemic haemodynamic changes within a fluid-filled, physical system. However, our current precise measurement of short-term cardiovascular fluctuations does not necessarily mean we have an adequate understanding of them. Empirical observation suggests that both respiratory and Mayer wave fluctuations derive from mutable autonomic and haemodynamic inputs. Evidence strongly suggests that respiratory sinus arrhythmia both contributes to and buffers respiratory arterial pressure fluctuations. Moreover, even though virtual abolition of all R-R interval variability by cholinergic blockade suggests that parasympathetic stimulation is essential for expression of these variabilities, respiratory sinus arrhythmia does not always reflect a purely vagal phenomenon. The arterial baroreflex has been cited as the mechanism for both respiratory and Mayer wave frequency fluctuations. However, data suggest that both cardiac vagal and vascular sympathetic fluctuations at these frequencies are independent of baroreflex mechanisms and, in fact, contribute to pressure fluctuations. Results from cardiovascular modelling can suggest possible sources for these rhythms. For example, modelling originally suggested low frequency cardiovascular rhythms derived from intrinsic delays in baroreceptor control, and experimental evidence subsequently corroborated this possibility. However, the complex stochastic relations between and variabilities in these rhythms indicate no single mechanism is responsible. If future study of cardiovascular variabilities is to move beyond qualitative suggestions of determinants to quantitative elucidation of critical physical mechanisms, both experimental design and model construction will have to be more trenchant.
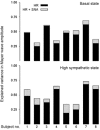
Figures

References
-
- Abbiw-Jackson RM, Langford WF. Gain-induced oscillations in blood pressure. Journal of Mathematical Biology. 1998;37:203–234. - PubMed
-
- Abdel-Rahman AR, Merrill RH, Wooles WR. Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. Journal of Applied Physiology. 1994;77:606–613. - PubMed
-
- Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: Investigation by spectral analysis. American Journal of Physiology. 1985;249:H867–875. - PubMed
-
- Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Respiratory modulation of human autonomic rhythms. American Journal of Physiology – Heart and Circulatory Physiology. 2001;280:H2674–2688. - PubMed
-
- Barbieri R, Bianchi AM, Triedman JK, Mainardi LT, Cerutti S, Saul JP. Model dependency of multivariate autoregressive spectral analysis. IEEE Engineering in Medicine and Biology. 1997;16:74–85. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

