The Ca(V)2.3 Ca(2+) channel subunit contributes to R-type Ca(2+) currents in murine hippocampal and neocortical neurones
- PMID: 12154172
- PMCID: PMC2290463
- DOI: 10.1113/jphysiol.2002.020677
The Ca(V)2.3 Ca(2+) channel subunit contributes to R-type Ca(2+) currents in murine hippocampal and neocortical neurones
Abstract
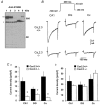
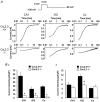
Different subtypes of voltage-dependent Ca(2+) currents in native neurones are essential in coupling action potential firing to Ca(2+) influx. For most of these currents, the underlying Ca(2+) channel subunits have been identified on the basis of pharmacological and biophysical similarities. In contrast, the molecular basis of R-type Ca(2+) currents remains controversial. We have therefore examined the contribution of the Ca(V)2.3 (alpha(1E)) subunits to R-type currents in different types of central neurones using wild-type mice and mice in which the Ca(V)2.3 subunit gene was deleted. In hippocampal CA1 pyramidal cells and dentate granule neurones, as well as neocortical neurones of wild-type mice, Ca(2+) current components resistant to the combined application of omega-conotoxin GVIA and MVIIC, omega-agatoxin IVa and nifedipine (I(Ca,R)) were detected that were composed of a large R-type and a smaller T-type component. In Ca(V)2.3-deficient mice, I(Ca,R) was considerably reduced in CA1 neurones (79 %) and cortical neurones (87 %), with less reduction occurring in dentate granule neurones (47 %). Analysis of tail currents revealed that the reduction of I(Ca,R) is due to a selective reduction of the rapidly deactivating R-type current component in CA1 and cortical neurones. In all cell types, I(Ca,R) was highly sensitive to Ni(2+) (100 microM: 71-86 % block). A selective antagonist of cloned Ca(V)2.3 channels, the spider toxin SNX-482, partially inhibited I(Ca,R) at concentrations up to 300 nM in dentate granule cells and cortical neurones (50 and 57 % block, EC(50) 30 and 47 nM, respectively). I(Ca,R) in CA1 neurones was significantly less sensitive to SNX-482 (27 % block, 300 nM SNX-482). Taken together, our results show clearly that Ca(V)2.3 subunits underlie a significant fraction of I(Ca,R) in different types of central neurones. They also indicate that Ca(V)2.3 subunits may give rise to Ca(2+) currents with differing pharmacological properties in native neurones.
Figures



Similar articles
-
Blocker-resistant Ca2+ currents in rat CA1 hippocampal pyramidal neurons.Neuroscience. 2003;116(3):629-38. doi: 10.1016/s0306-4522(02)00777-7. Neuroscience. 2003. PMID: 12573706
-
The status of voltage-dependent calcium channels in alpha 1E knock-out mice.J Neurosci. 2000 Dec 1;20(23):8566-71. doi: 10.1523/JNEUROSCI.20-23-08566.2000. J Neurosci. 2000. PMID: 11102459 Free PMC article.
-
High expression of the R-type voltage-gated Ca2+ channel and its involvement in Ca2+-dependent gonadotropin-releasing hormone release in GT1-7 cells.Endocrinology. 2004 May;145(5):2375-83. doi: 10.1210/en.2003-1257. Epub 2004 Jan 21. Endocrinology. 2004. PMID: 14736732
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
-
Pathophysiological significance of T-type Ca2+ channels: expression of T-type Ca2+ channels in fetal and diseased heart.J Pharmacol Sci. 2005 Nov;99(3):205-10. doi: 10.1254/jphs.fmj05002x3. Epub 2005 Nov 1. J Pharmacol Sci. 2005. PMID: 16272790 Review.
Cited by
-
Genetic findings in sport-related concussions: potential for individualized medicine?Concussion. 2017 Jan 24;2(1):CNC26. doi: 10.2217/cnc-2016-0020. eCollection 2017 Mar. Concussion. 2017. PMID: 30202567 Free PMC article. Review.
-
Muscarinic enhancement of R-type calcium currents in hippocampal CA1 pyramidal neurons.J Neurosci. 2006 Jun 7;26(23):6249-58. doi: 10.1523/JNEUROSCI.1009-06.2006. J Neurosci. 2006. PMID: 16763032 Free PMC article.
-
What is the core oscillator in the speract-activated pathway of the Strongylocentrotus purpuratus sperm flagellum?Biophys J. 2012 Jun 6;102(11):2481-8. doi: 10.1016/j.bpj.2012.03.075. Epub 2012 Jun 5. Biophys J. 2012. PMID: 22713563 Free PMC article.
-
Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy.J Neurosci. 2008 Dec 3;28(49):13341-53. doi: 10.1523/JNEUROSCI.1421-08.2008. J Neurosci. 2008. PMID: 19052226 Free PMC article.
-
Apamin Boosting of Synaptic Potentials in CaV2.3 R-Type Ca2+ Channel Null Mice.PLoS One. 2015 Sep 29;10(9):e0139332. doi: 10.1371/journal.pone.0139332. eCollection 2015. PLoS One. 2015. PMID: 26418566 Free PMC article.
References
-
- Beck H, Steffens R, Heinemann U, Elger CE. Properties of voltage-activated calcium currents in acutely dissociated human hippocampal granule cells. Journal of Neurophysiology. 1998;77:1526–1537. - PubMed
-
- Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, Mori Y, SchwartZ A, Snutch TP, Tanabe T, et al. The naming of voltage-gated calcium channels. Neuron. 1994;13:505–506. - PubMed
-
- Burley JR, Dolphin AC. Overlapping selectivity of neurotoxin and dihydropyridine calcium channel blockers in cerebellar granule neurones. Neuropharmacology. 2000;39:1740–1755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

