Carbachol triggers RyR-dependent Ca(2+) release via activation of IP(3) receptors in isolated rat gastric myocytes
- PMID: 12154174
- PMCID: PMC2290441
- DOI: 10.1113/jphysiol.2002.020131
Carbachol triggers RyR-dependent Ca(2+) release via activation of IP(3) receptors in isolated rat gastric myocytes
Abstract
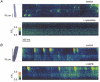
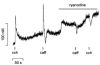
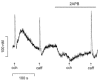
Possible interactions between different intracellular Ca(2+) release channels were studied in isolated rat gastric myocytes using agonist-evoked Ca(2+) signals. Spontaneous, local Ca(2+) transients were observed in fluo-4-loaded cells with linescan confocal imaging. These were blocked by ryanodine (100 microM) but not by the inositol 1,4,5-trisphosphate receptor (IP(3)R) blocker, 2-aminoethoxydiphenyl borate (100 microM), identifying them as Ca(2+) sparks. Caffeine (10 mM) and carbachol (10 microM) initiated Ca(2+) release at sites which co-localized with each other and with any Ca(2+) spark sites. In fura-2-loaded cells extracellular 2-aminoethoxydiphenyl borate and intracellular heparin (5 mg ml(-1)) both inhibited the global cytoplasmic [Ca(2+)] transient evoked by carbachol, confirming that it was IP(3)R-dependent. 2-Aminoethoxydiphenyl borate and heparin also increased the response to caffeine. This probably reflected an increased Ca(2+) store content since 2-aminoethoxydiphenyl borate more than doubled the amplitude of transients evoked by ionomycin. Ryanodine completely abolished carbachol and caffeine responses but only reduced ionomycin transients by 30 %, suggesting that blockade of carbachol transients by ryanodine was not simply due to store depletion. Double labelling of IP(3)Rs and RyRs demonstrated extensive overlap in their distribution. These results suggest that carbachol stimulates Ca(2+) release through co-operation between IP(3)Rs and RyRs, and implicate IP(3)Rs in the regulation of Ca(2+) store content.
Figures




Similar articles
-
Regulation of muscarinic cationic current in myocytes from guinea-pig ileum by intracellular Ca2+ release: a central role of inositol 1,4,5-trisphosphate receptors.Cell Calcium. 2004 Nov;36(5):367-86. doi: 10.1016/j.ceca.2004.02.021. Cell Calcium. 2004. PMID: 15451621
-
Mechanisms underlying activation of transient BK current in rabbit urethral smooth muscle cells and its modulation by IP3-generating agonists.Am J Physiol Cell Physiol. 2013 Sep 15;305(6):C609-22. doi: 10.1152/ajpcell.00025.2013. Epub 2013 Jun 26. Am J Physiol Cell Physiol. 2013. PMID: 23804200 Free PMC article.
-
The phospholipase C inhibitor U-73122 inhibits Ca(2+) release from the intracellular sarcoplasmic reticulum Ca(2+) store by inhibiting Ca(2+) pumps in smooth muscle.Br J Pharmacol. 2010 Jul;160(6):1295-301. doi: 10.1111/j.1476-5381.2010.00771.x. Br J Pharmacol. 2010. PMID: 20590621 Free PMC article.
-
Interactions between inositol 1,4,5-trisphosphate receptors and ryanodine receptors in smooth muscle: one store or two?Cell Calcium. 2004 Jun;35(6):613-9. doi: 10.1016/j.ceca.2004.01.016. Cell Calcium. 2004. PMID: 15110151 Review.
-
Sarcolemma agonist-induced interactions between InsP3 and ryanodine receptors in Ca2+ oscillations and waves in smooth muscle.Biochem Soc Trans. 2003 Oct;31(Pt 5):920-4. doi: 10.1042/bst0310920. Biochem Soc Trans. 2003. PMID: 14505449 Review.
Cited by
-
Involvement of presenilin holoprotein upregulation in calcium dyshomeostasis of Alzheimer's disease.J Cell Mol Med. 2013 Feb;17(2):293-302. doi: 10.1111/jcmm.12008. Epub 2013 Feb 5. J Cell Mol Med. 2013. PMID: 23379308 Free PMC article.
-
Sub-plasmalemmal [Ca2+]i upstroke in myocytes of the guinea-pig small intestine evoked by muscarinic stimulation: IP3R-mediated Ca2+ release induced by voltage-gated Ca2+ entry.Cell Calcium. 2008 Feb;43(2):122-41. doi: 10.1016/j.ceca.2007.04.012. Epub 2007 Jun 13. Cell Calcium. 2008. PMID: 17570487 Free PMC article.
-
cAMP/PKA-dependent increases in Ca Sparks, oscillations and SR Ca stores in retinal arteriolar myocytes after exposure to vasopressin.Invest Ophthalmol Vis Sci. 2010 Mar;51(3):1591-8. doi: 10.1167/iovs.09-4401. Epub 2009 Dec 3. Invest Ophthalmol Vis Sci. 2010. PMID: 19959643 Free PMC article.
-
Agonist-evoked Ca(2+) wave progression requires Ca(2+) and IP(3).J Cell Physiol. 2010 Aug;224(2):334-44. doi: 10.1002/jcp.22103. J Cell Physiol. 2010. PMID: 20432430 Free PMC article.
-
Ryanodine receptors selectively contribute to the formation of taste-evoked calcium signals in mouse taste cells.Eur J Neurosci. 2010 Dec;32(11):1825-35. doi: 10.1111/j.1460-9568.2010.07463.x. Epub 2010 Oct 19. Eur J Neurosci. 2010. PMID: 20955474 Free PMC article.
References
-
- Arnaudeau S, Boittin FX, MacreZ N, Lavie JL, Mironneau C, Mironneau J. L-type and Ca2+ release channel-dependent hierarchical Ca2+ signalling in rat portal vein myocytes. Cell Calcium. 1997;22:399–411. - PubMed
-
- Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. American Journal of Physiology – Cell Physiology. 2000;279:C126–135. - PubMed
-
- Bayguinov O, Hagen B, Sanders KM. Muscarinic stimulation increases basal Ca2+ and inhibits spontaneous Ca2+ transients in murine colonic myocytes. American Journal of Physiology – Cell Physiology. 2001;280:C689–700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

