A stretch-activated anion channel is up-regulated by the malaria parasite Plasmodium falciparum
- PMID: 12154179
- PMCID: PMC2290452
- DOI: 10.1113/jphysiol.2002.022970
A stretch-activated anion channel is up-regulated by the malaria parasite Plasmodium falciparum
Abstract
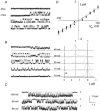
A recent study on malaria-infected human red blood cells (RBCs) has shown induced ion channel activity in the host cell membrane, but the questions of whether they are host- or parasite-derived and their molecular nature have not been resolved. Here we report a comparison of a malaria-induced anion channel with an endogenous anion channel in Plasmodium falciparum-infected human RBCs. Ion channel activity was measured using the whole-cell, cell-attached and excised inside-out configurations of the patch-clamp method. Parasitised RBCs were cultured in vitro, using co-cultured uninfected RBCs as controls. Unstimulated uninfected RBCs possessed negligible numbers of active anion channels. However, anion channels could be activated in the presence of protein kinase A (PKA) and ATP in the pipette solution or by membrane deformation. These channels displayed linear conductance (~15 pS), were blocked by known anion channel inhibitors and showed the permeability sequence I(-) > Br(-) > Cl(-). In addition, in less than 5 % of excised patches, an outwardly rectifying anion channel (~80 pS, outward conductance) was spontaneously active. The host membrane of malaria-infected RBCs possessed spontaneously active anion channel activity, with identical conductances, pharmacology and selectivity to the linear conductance channel measured in stimulated uninfected RBCs. Furthermore, the channels measured in malaria-infected RBCs were shown to have a low open-state probability (P(o)) at positive potentials, which explains the inward rectification of membrane conductance observed when using the whole-cell configuration. The data are consistent with the presence of two endogenous anion channels in human RBCs, of which one (the linear conductance channel) is up-regulated by the malaria parasite P. falciparum.
Figures


References
-
- Busse R, Ogilvie A, Pohl U. Vasomotor activity of diadenosine triphosphate and diadenosine tetraphosphate in isolated arteries. American Journal of Physiology. 1988;254:H823–828. - PubMed
-
- Chishti AH, Maalouf GJ, Marfatia S, Palek J, Wang W, Fisher D, Liu S. Phosphorylation of Protein 4. 1 in. Plasmodium falciparum-infected human red blood cells. Blood. 1994;83:3339–3345. - PubMed
-
- Desai SA, Bezrukov SM, Zimmerberg J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature. 2000;406:1001–1005. - PubMed
-
- Doerig CD, Doerig CM, Horrocks P, Carlton J, Sultan A, Arnot D, Carter R. Pfcrk-1, a developmentally regulated cdc2-related protein kinase of Plasmodium falciparum. Molecular and Biochemical Parasitology. 1995;70:167–174. - PubMed
-
- Egée S, Lapaix F, Cossins AR, Thomas SLY. The role of anion and cation channels in volume regulatory responses in trout red blood cells. Bioelectrochemistry. 2000;52:133–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

