Regulation of an ATP-conductive large-conductance anion channel and swelling-induced ATP release by arachidonic acid
- PMID: 12154180
- PMCID: PMC2290458
- DOI: 10.1113/jphysiol.2002.019802
Regulation of an ATP-conductive large-conductance anion channel and swelling-induced ATP release by arachidonic acid
Abstract
Mouse mammary C127 cells responded to hypotonic stimulation with activation of the volume-dependent ATP-conductive large conductance (VDACL) anion channel and massive release of ATP. Arachidonic acid downregulated both VDACL currents and swelling-induced ATP release in the physiological concentration range with K(d) of 4- 6 microM. The former effect observed in the whole-cell or excised patch mode was more prominent than the latter effect observed in intact cells. The arachidonate effects were direct and not mediated by downstream metabolic products, as evidenced by their insensitivity to inhibitors of arachidonate-metabolizing oxygenases, and by the observation that they were mimicked by cis-unsaturated fatty acids, which are not substrates for oxygenases. A membrane-impermeable analogue, arachidonyl coenzyme A was effective only from the cytosolic side of membrane patches suggesting that the binding site is localized intracellularly. Non-charged arachidonate analogues as well as trans-unsaturated and saturated fatty acids had no effect on VDACL currents and ATP release, indicating the importance of arachidonate's negative charge and specific hydrocarbon chain conformation in the inhibitory effect. VDACL anion channels were inhibited by arachidonic acid in two different ways: channel shutdown (K(d) of 4- 5 microM) and reduced unitary conductance (K(d) of 13-14 microM) without affecting voltage dependence of open probability. ATP(4-)-conducting inward currents measured in the presence of 100 mM ATP in the bath were reversibly inhibited by arachidonic acid. Thus, we conclude that swelling-induced ATP release and its putative pathway, the VDACL anion channel, are under a negative control by intracellular arachidonic acid signalling in mammary C127 cells.
Figures


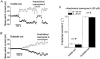

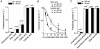

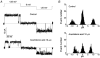
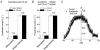


Similar articles
-
Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release.J Gen Physiol. 2001 Sep;118(3):251-66. doi: 10.1085/jgp.118.3.251. J Gen Physiol. 2001. PMID: 11524456 Free PMC article.
-
Volume-regulatory Cl- channel currents in cultured human epithelial cells.J Physiol. 1992 Oct;456:351-71. doi: 10.1113/jphysiol.1992.sp019340. J Physiol. 1992. PMID: 1284079 Free PMC article.
-
Arachidonic acid activation of a new family of K+ channels in cultured rat neuronal cells.J Physiol. 1995 May 1;484 ( Pt 3)(Pt 3):643-60. doi: 10.1113/jphysiol.1995.sp020693. J Physiol. 1995. PMID: 7623282 Free PMC article.
-
ATP-conducting maxi-anion channel: a new player in stress-sensory transduction.Jpn J Physiol. 2004 Feb;54(1):7-14. doi: 10.2170/jjphysiol.54.7. Jpn J Physiol. 2004. PMID: 15040843 Review.
-
Direct regulation of ion channels by fatty acids.Trends Neurosci. 1991 Mar;14(3):96-100. doi: 10.1016/0166-2236(91)90069-7. Trends Neurosci. 1991. PMID: 1709540 Review.
Cited by
-
Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway.Biophys J. 2004 Sep;87(3):1672-85. doi: 10.1529/biophysj.104.043174. Biophys J. 2004. PMID: 15345546 Free PMC article.
-
X-ray crystallographic studies of the extracellular domain of the first plant ATP receptor, DORN1, and the orthologous protein from Camelina sativa.Acta Crystallogr F Struct Biol Commun. 2016 Oct 1;72(Pt 10):782-787. doi: 10.1107/S2053230X16014278. Epub 2016 Sep 22. Acta Crystallogr F Struct Biol Commun. 2016. PMID: 27710944 Free PMC article.
-
Synthesis and antiplatelet activity of antithrombotic thiourea compounds: biological and structure-activity relationship studies.Molecules. 2015 Apr 20;20(4):7174-200. doi: 10.3390/molecules20047174. Molecules. 2015. PMID: 25903367 Free PMC article.
-
The maxi-anion channel: a classical channel playing novel roles through an unidentified molecular entity.J Physiol Sci. 2009 Jan;59(1):3-21. doi: 10.1007/s12576-008-0008-4. Epub 2008 Dec 9. J Physiol Sci. 2009. PMID: 19340557 Free PMC article. Review.
-
Protective mechanism of shenmai on myocardial ischemia-reperfusion through the energy metabolism pathway.Am J Transl Res. 2019 Jul 15;11(7):4046-4062. eCollection 2019. Am J Transl Res. 2019. PMID: 31396317 Free PMC article.
References
-
- Basavappa SS, Pedersen F, Jorgensen NK, Ellory JC, Hoffmann EK. Swelling-induced arachidonic acid release via the 85-kDa cPLA2 in human neuroblastoma cells. Journal of Neurophysiology. 1998;79:1441–1449. - PubMed
-
- Bell PD, Lapointe J-Y, Sabirov R, Hayashi S, Okada Y. Maxi-chloride channel in macula densa cells: possible pathway for ATP release. FASEB Journal. 2000;14:A134.
-
- Bodas E, Aleu J, Pujol G, Martin-Satue M, Marsal J, Solsona C. ATP crossing the cell plasma membrane generates an ionic current in Xenopus oocytes. Journal of Biological Chemistry. 2000;275:20268–20273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

