Passive water and urea permeability of a human Na(+)-glutamate cotransporter expressed in Xenopus oocytes
- PMID: 12154181
- PMCID: PMC2290454
- DOI: 10.1113/jphysiol.2002.020586
Passive water and urea permeability of a human Na(+)-glutamate cotransporter expressed in Xenopus oocytes
Abstract
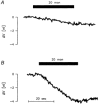
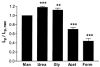
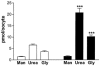
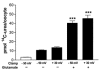
The human Na(+)-glutamate transporter (EAAT1) was expressed in Xenopus laevis oocytes. The passive water permeability, L(p), was derived from volume changes of the oocyte induced by changes in the external osmolarity. Oocytes were subjected to two-electrode voltage clamp. In the presence of Na(+), the EAAT1-specific (defined in Discussion) L(p) increased linearly with positive clamp potentials, the L(p) being around 23 % larger at +50 mV than at -50 mV. L-Glutamate increased the EAAT1-specific L(p) by up to 40 %. The K(0.5) for the glutamate-dependent increase was 20 +/- 6 microM, which is similar to the K(0.5) value for glutamate activation of transport. The specific inhibitor DL-threo-beta-benzyloxyaspartate (TBOA) reduced the EAAT1-specific L(p) to 72 %. EAAT1 supported passive fluxes of [(14)C]urea and [(14)C]glycerol. The [(14)C]urea flux was increased in the presence of glutamate. The data suggest that the permeability depends on the conformational equilibrium of the EAAT1. At positive potentials and in the presence of Na(+) and glutamate, the pore is enlarged and water and urea penetrate more readily. The L(p) was larger when measured with urea or glycerol as osmolytes as compared with mannitol. Apparently, the properties of the pore are not uniform along its length. The outer section may accommodate urea and glycerol in an osmotically active form, giving rise to larger water fluxes. The physiological role of EAAT1 for water homeostasis in the central nervous system is discussed.
Figures



Similar articles
-
Water transport by the human Na+-coupled glutamate cotransporter expressed in Xenopus oocytes.J Physiol. 2001 Feb 1;530(Pt 3):367-78. doi: 10.1111/j.1469-7793.2001.0367k.x. J Physiol. 2001. PMID: 11158269 Free PMC article.
-
Water and urea permeation pathways of the human excitatory amino acid transporter EAAT1.Biochem J. 2011 Oct 15;439(2):333-40. doi: 10.1042/BJ20110905. Biochem J. 2011. PMID: 21732909
-
Up-Regulation of Excitatory Amino Acid Transporters EAAT1 and EAAT2 by ß-Klotho.Neurosignals. 2015;23(1):59-70. doi: 10.1159/000442604. Epub 2015 Dec 19. Neurosignals. 2015. PMID: 26684854
-
Molecular water pumps.Rev Physiol Biochem Pharmacol. 2000;141:97-151. doi: 10.1007/BFb0119578. Rev Physiol Biochem Pharmacol. 2000. PMID: 10916424 Review.
-
Cotransporters as molecular water pumps.Int Rev Cytol. 2002;215:259-84. doi: 10.1016/s0074-7696(02)15012-1. Int Rev Cytol. 2002. PMID: 11952231 Review.
Cited by
-
Glial K⁺ clearance and cell swelling: key roles for cotransporters and pumps.Neurochem Res. 2012 Nov;37(11):2299-309. doi: 10.1007/s11064-012-0731-3. Epub 2012 Feb 26. Neurochem Res. 2012. PMID: 22367475 Review.
-
Water permeability of Na+-K+-2Cl- cotransporters in mammalian epithelial cells.J Physiol. 2005 Oct 1;568(Pt 1):123-35. doi: 10.1113/jphysiol.2005.093526. Epub 2005 Jul 14. J Physiol. 2005. PMID: 16020454 Free PMC article.
-
Water permeation through the sodium-dependent galactose cotransporter vSGLT.Biophys J. 2010 Oct 6;99(7):L56-8. doi: 10.1016/j.bpj.2010.08.055. Biophys J. 2010. PMID: 20923633 Free PMC article.
-
The Split Personality of Glutamate Transporters: A Chloride Channel and a Transporter.Neurochem Res. 2016 Mar;41(3):593-9. doi: 10.1007/s11064-015-1699-6. Epub 2015 Aug 25. Neurochem Res. 2016. PMID: 26303507
-
Oligomeric structure and functional characterization of the urea transporter from Actinobacillus pleuropneumoniae.J Mol Biol. 2009 Apr 3;387(3):619-27. doi: 10.1016/j.jmb.2009.02.005. Epub 2009 Feb 11. J Mol Biol. 2009. PMID: 19361419 Free PMC article.
References
-
- Auger C, Attwell D. Fast removal of synaptic glutamate by postsynaptic transporters. Neuron. 2000;28:547–558. - PubMed
-
- Barbour B, Brew H, Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988;335:433–435. - PubMed
-
- Borgnia MJ, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annual Review of Biochemistry. 1999;68:425–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

